Template:Kyoto/Notebook/Sep 24
From 2013.igem.org
(Difference between revisions)
(→Transformation) |
(→Ligation) |
||
| (26 intermediate revisions not shown) | |||
| Line 41: | Line 41: | ||
|17||9/20 Plac-aptamer12-1R-DT 4 | |17||9/20 Plac-aptamer12-1R-DT 4 | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_0924_E1.jpg]]<br> |
</div> | </div> | ||
| Line 78: | Line 78: | ||
===Liquid Culture=== | ===Liquid Culture=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author">No name </span> | + | <span class="author">No name</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Sample||medium | !Sample||medium | ||
| Line 85: | Line 85: | ||
|} | |} | ||
</div> | </div> | ||
| + | |||
| + | ===Restriction Enzyme Digestion(PCR product)=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | Concentrate PCR product by evaporator until volume become less than 1µL, add all sulution and pipetting. </div> | ||
| + | {| class="wikitable" | ||
| + | !||10*cut smart buffer||BsaI-HF||MilliQ||total | ||
| + | |- | ||
| + | |9/22 Pcon-pT181 attenuator (E-0) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-pT181 attenuator (2-3A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 spinach-DT(3-2) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Ptet-pT181 antisense (1-3) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-tet aptamer-DT (E-1A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-spinach-DT (E-1A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-pT181 attenuator (E-1A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 DT (1-3) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 tet aptamer-DT (1-3) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-pT181 attenuator (1-SA) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-RBS-tetR-DT (1-2A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-RBS-tetR-DT (3-2A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-RBS-tetR-DT (E-2A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-RBS-GFP-DT (1-2A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/22 Pcon-RBS-GFP-DT (2-SA) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/23 Pcon-pT181 attenuator-DT (E-1A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/23 Pcon-pT181 attenuator-DT (0-1A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/23 Pcon-pT181 antisense-spinach-DT (1-2A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/23 Pcon-pT181 antisense (1-2A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/23 Ptet-1 (2-S) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |- | ||
| + | |9/23 Plac-2 (E-1A) ||2.0 µL ||0.3 µL ||17 µL ||19.3µL | ||
| + | |} | ||
| + | Incubate 37°C 12hour</div> | ||
| + | |||
| + | ===Column Refining=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !Name||concentration[µg/mL]||260/280||260/230 | ||
| + | |- | ||
| + | |8/22 Pcon-pT181 attenuator(E-0)||7.8||1.70||0.03 | ||
| + | |- | ||
| + | |8/22 Pcon-pT181 attenuator(2-SA)||17.3||1.93||0.03 | ||
| + | |- | ||
| + | |8/22 spinach-DT (3-2)||22.4||1.93||0.09 | ||
| + | |- | ||
| + | |8/22 Ptet-PT181 anisense (1-3)||18.3||1.58||0.14 | ||
| + | |- | ||
| + | |8/22 Pcon-aptamer12_1R-DT (E-1A)||8.2||2.10||0.05 | ||
| + | |- | ||
| + | |8/22 Pcon-spinach-DT(E-1A)||5.6||0.91||0.05 | ||
| + | |- | ||
| + | |8/22 Pcon-pT181 attenuator(E-1A)||18.5||1.80||0.12 | ||
| + | |- | ||
| + | |8/22 DT(1-3)||7.3||1.13||0.04 | ||
| + | |- | ||
| + | |8/22 tetR aptamer 12_1R-DT(1-3)||14.6||1.74||0.06 | ||
| + | |- | ||
| + | |8/22 Pcon-pT181 attenuator(1-SA)||7.9||1.57||0.04 | ||
| + | |- | ||
| + | |8/22 Pcon-RBS-tetR-DT(1-2Ⓐ)||41.6||1.81||0.25 | ||
| + | |- | ||
| + | |8/22 Pcon-RBS-tetR-DT(3-2Ⓐ)||30.2||1.75||0.15 | ||
| + | |- | ||
| + | |8/22 Pcon-RBS-tetR-DT(E-2Ⓐ)||36.9||1.75||0.56 | ||
| + | |- | ||
| + | |8/22 Pcon-RBS-GFP-DT(1-SⒶ)||43.5||1.83||0.33 | ||
| + | |- | ||
| + | |8/22 Pcon-RBS-GFP-DT(2-SⒶ)||64.6||1.82||0.30 | ||
| + | |- | ||
| + | |8/23 Pcon-pT181 attenuator-DT (E-1A)||31.2||1.83||0.09 | ||
| + | |- | ||
| + | |8/23 Pcon-pT181 attenuator-DT (0-1A)||64.5||1.80||0.43 | ||
| + | |- | ||
| + | |8/23 Pcon-pT181 antisense-spinach-DT (1-2A)||36.4||1.88||0.21 | ||
| + | |- | ||
| + | |8/23 Pcon-pT181 antisense-spinach-DT (E-1A)||27.9||1.92||0.16 | ||
| + | |- | ||
| + | |8/23 Ptet(1)(2-S)||37.0||1.91||0.16 | ||
| + | |- | ||
| + | |9/23 Plac-2(E-1A) ||21.2||1.82||0.13 | ||
| + | |} | ||
===Transformation=== | ===Transformation=== | ||
| Line 111: | Line 211: | ||
</div> | </div> | ||
| - | === Digestion=== | + | ===Restriction Enzyme Digestion=== |
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Kojima </span> | <span class="author">Kojima </span> | ||
| Line 157: | Line 257: | ||
|7|| pSB1C3 ||--||-- | |7|| pSB1C3 ||--||-- | ||
|}<br> | |}<br> | ||
| - | [[File: | + | [[File:Igku_0924_E2.jpg]]<br> |
<br> | <br> | ||
</div> | </div> | ||
| + | |||
===Miniprep=== | ===Miniprep=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| Line 174: | Line 275: | ||
<span class="author">Kojima</span> | <span class="author">Kojima</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !Lane||DNA|| | + | !Lane||DNA||Enzyme1||Enzyme2 |
|- | |- | ||
|1||100kb ladder||--||-- | |1||100kb ladder||--||-- | ||
| Line 200: | Line 301: | ||
|12|| pSB1C3||EcoRI||SpeI | |12|| pSB1C3||EcoRI||SpeI | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0924_G1.jpg]]<br> |
| - | [[File: | + | [[File:Igku_0924_G2.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
| Line 322: | Line 423: | ||
|17||9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 8 | |17||9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 8 | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_0924_E3.jpg]]<br> |
</div> | </div> | ||
| Line 356: | Line 457: | ||
|14||-- | |14||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:igku_0924_E4.jpg]]<br> |
</div> | </div> | ||
| Line 363: | Line 464: | ||
<span class="author">Nakamoto</span> | <span class="author">Nakamoto</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !9/24 aptamer12-1R-DT ||EcoRI||XbaI || buffer||BSA||MilliQ||total | + | !||9/24 aptamer12-1R-DT ||EcoRI||XbaI || buffer||BSA||MilliQ||total |
|- | |- | ||
|2cuts||17.5µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||4.5µL ||30µL | |2cuts||17.5µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||4.5µL ||30µL | ||
| Line 370: | Line 471: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !9/20 Ptet||SpeI||PstI || buffer||BSA||MilliQ||total | + | !||9/20 Ptet||SpeI||PstI || buffer||BSA||MilliQ||total |
|- | |- | ||
|2cuts||9.5µL||1.0µL ||1.0µL ||3.0µL ||0µL ||15.5µL ||30µL | |2cuts||9.5µL||1.0µL ||1.0µL ||3.0µL ||0µL ||15.5µL ||30µL | ||
| Line 377: | Line 478: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !9/22 RBS-GFP-DT||EcoRI ||SpeI || buffer||BSA||MilliQ||total | + | !||9/22 RBS-GFP-DT||EcoRI ||SpeI || buffer||BSA||MilliQ||total |
|- | |- | ||
|2cuts||3.8µL||1.0µL ||1.0µL ||3.0µL ||0µL ||21.2µL ||30µL | |2cuts||3.8µL||1.0µL ||1.0µL ||3.0µL ||0µL ||21.2µL ||30µL | ||
| Line 384: | Line 485: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !9/22 Pcon-RBS-GFP-DT||EcoRI ||XbaI || buffer||BSA||MilliQ||total | + | !||9/22 Pcon-RBS-GFP-DT||EcoRI ||XbaI || buffer||BSA||MilliQ||total |
|- | |- | ||
|2cuts||2.4µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||19.6µL ||30µL | |2cuts||2.4µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||19.6µL ||30µL | ||
| Line 391: | Line 492: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !9/22 Pcon-pT181attenuator 1 ||EcoRI ||XbaI || buffer||BSA||MilliQ||total | + | !||9/22 Pcon-pT181attenuator 1 ||EcoRI ||XbaI || buffer||BSA||MilliQ||total |
|- | |- | ||
|2cuts||5.4µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||16.6µL ||30µL | |2cuts||5.4µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||16.6µL ||30µL | ||
| Line 398: | Line 499: | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | !9/22 RBS-GFP-DT||XbaI ||PstI || buffer||BSA||MilliQ||total | + | !||9/22 RBS-GFP-DT||XbaI ||PstI || buffer||BSA||MilliQ||total |
|- | |- | ||
|2cuts||3.8µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||18.2µL ||30µL | |2cuts||3.8µL||1.0µL ||1.0µL ||3.0µL ||3.0µL ||18.2µL ||30µL | ||
|- | |- | ||
|NC||0.2µL ||0µL ||0µL ||1.0µL ||1.0µL ||7.8µL ||10µL | |NC||0.2µL ||0µL ||0µL ||1.0µL ||1.0µL ||7.8µL ||10µL | ||
| - | |} | + | |} |
===Ligation=== | ===Ligation=== | ||
| Line 411: | Line 512: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |||9/22 aptamer12-1R-DT(EcoRI&XbaI) ||1.4 µL||9/24 Pcon-pT181attenuator(EcoRI&SpeI)||26 µL||3.5 µL | + | |experiment||9/22 aptamer12-1R-DT(EcoRI&XbaI) ||1.4 µL||9/24 Pcon-pT181attenuator(EcoRI&SpeI)||26 µL||3.5 µL |
|- | |- | ||
| - | |||9/4 Ptet(SpeI&PstI)||2.0 µL||9/21 RBS-GFP-DT(XbaI&PstI)||9.0 µL||3.5 µL | + | |experiment||9/4 Ptet(SpeI&PstI)||2.0 µL||9/21 RBS-GFP-DT(XbaI&PstI)||9.0 µL||3.5 µL |
|} | |} | ||
</div> | </div> | ||
| Line 508: | Line 609: | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1kb ladder||--||-- | + | |1||1kb ladder||--||-- |
|- | |- | ||
|2||9/24 aptamer12-1R-DT||EcoRI||XbaI | |2||9/24 aptamer12-1R-DT||EcoRI||XbaI | ||
| Line 520: | Line 621: | ||
|6||9/24 RBS-GFP-DT ||EcoRI||SpeI | |6||9/24 RBS-GFP-DT ||EcoRI||SpeI | ||
|- | |- | ||
| - | |7| 9/24 RBS-GFP-DT ||-- ||-- | + | |7||9/24 RBS-GFP-DT ||-- ||-- |
|- | |- | ||
|8||9/24 Pcon-RBS-GFP-DT ||EcoRI||XbaI | |8||9/24 Pcon-RBS-GFP-DT ||EcoRI||XbaI | ||
| Line 536: | Line 637: | ||
|13||9/24 RBS-GFP-DT ||--||-- | |13||9/24 RBS-GFP-DT ||--||-- | ||
|- | |- | ||
| - | |14||1kb ladder | + | |14||1kb ladder||--||-- |
|} | |} | ||
| - | [[File: | + | [[File:Igku_0924_E5.jpg]]<br> |
===Gel Extraction=== | ===Gel Extraction=== | ||
| Line 571: | Line 672: | ||
|12||RBS-GFP-DT||EcoRI||SpeI | |12||RBS-GFP-DT||EcoRI||SpeI | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0924_G3.jpg]]<br> |
| - | [[File: | + | [[File:Igku_0924_G4.jpg]]<br> |
{| class="wikitable" | {| class="wikitable" | ||
| Line 601: | Line 702: | ||
|12||RBS-GFP-DT||XbaI||PstI | |12||RBS-GFP-DT||XbaI||PstI | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0924_G5.jpg]]<br> |
| - | [[File: | + | [[File:Igku_0924_G6.jpg]]<br> |
<br> | <br> | ||
| Line 628: | Line 729: | ||
|8||1kb ladder | |8||1kb ladder | ||
|} | |} | ||
| - | |||
<br> | <br> | ||
| Line 651: | Line 751: | ||
|8||1kb ladder | |8||1kb ladder | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0924_E6.jpg]]<br> |
<br> | <br> | ||
| Line 674: | Line 774: | ||
|8|| Pcon-pT181attenuator-aptamer12-1R-DT 2 | |8|| Pcon-pT181attenuator-aptamer12-1R-DT 2 | ||
|} | |} | ||
| - | |||
<br> | <br> | ||
| Line 696: | Line 795: | ||
|- | |- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0924_E7.jpg]]<br> |
| + | |||
| + | ===Ligation=== | ||
| + | |||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter1||colspan="2"|Inserter2||colspan="2"|Inserter3||colspan="2"|Inserter4||(NEB)T4ligase||(NEB)Buffer||milliQ||total | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||tetR-aptamer12-1R-DT(1-3)||1 µL||Ptet(2-S)||0.3µL||Pcon-tetR-aptamer12-1R-DT(3-2A)||1.1µL||Plac(E-1A)||0.5µL||0.5µL||0.5µL||0.2 µL||5µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-pT181attenuator(E-1A)||1 µL||Pcon-tetR-aptamer12-1R-DT(1-2A)||1µL||Ptet(2-S)||0.3µL||-- ||-- ||0.5µL||0.5µL||0.7 µL||5µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-tetRaptamer12-1R-DT(E-1A)||2µL||Pcon-tetRaptamer12-1R-DT(1-2A)||1µL||Ptet(2-S)||0.3µL||--||--||0.5µL||0.5µL||0 µL||5.3µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-spinach-DT(E-1A)||3µL||Pcon-tetRaptamer12-1R-DT(1-2A)||1µL||Ptet(2-S)||0.3µL||--||--||0.5µL||0.5µL||0 µL||6.3µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-pT181antisense-DT(E-1A)||0.5µL||DT(1-3)||2µL||Ptet(2-S)||0.3µL||Pcon-tetRaptamer12-1R-DT(3-2A)||1.1µL||0.5µL||0.5µL||0.1 µL||5µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-pT181attenuator(E-1A)||1µL||tetRaptamer12-1R-DT(1-3)||1µL||Ptet(2-S)||0.3µL||Pcon-tetRaptamer12-1R-DT(3-2A)||1.1µL||0.5µL||0.5µL||0µL||5.4µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Ptet(2-S)||0.3µL||Pcon-tetRaptamer12-1R-DT(E-2A)||1µL||--||--||--||--||0.5µL||0.5µL||1.7µL||5µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-GFP-DT(2-SA)||0.5µL||Pcon-tetRaptamer12-1R-DT(E-2A)|1µL||--||--||--||--||--||0.5µL||0.5µL||1.5µL||5µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-tetRaptamer12-1R-DT(E-1A)||2µL||Pcon-pT181attenuator(1-SA)||2.1µL||--||--||--||--||0.5µL||0.5µL||0µL||6.1µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-pT181attenuator-DT(E-1A)||1µL||Pcon-pT181attenuator(1-SA)||2.1µL||--||--||--||--||0.5µL||0.5µL||0µL||5.1µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-tetR12-1R-DT(E-1A)||2µL||Pcon-GFP-DT(2-SA)||0.5µL||spinach-DT(3-2)||1µL||Ptet-pT181antisense(1-3)||1µL||0.5µL||0.5µL||0µL||6.5µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-tetRaptamer12-1R-DT(E-1A)||2µL||Pcon-pT181antisense(1-2A)||0.7µL||Pcon-pT181attenuator(2-SA)||2.1µL||--||--||0.5µL||0.5µL||0µL||6.8µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT(pSB1C3)||1µL||Pcon-tetRaptamer12-1R-DT(E-1A)||2µL||Pcon-pT181attenuator(2-SA)||2.1µL||Spinach-DT(3-2)||1µL||Ptet-pT181antisense(1-3)||1µL||0.5µL||0.5µL||0µL||8.1µL | ||
| + | |} | ||
| + | <br>Concentrate total (more than 5µL) by evaporater until volume become less than 5 µL<br> | ||
| + | </div> | ||
| + | |||
| + | ===PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !genome DNA||KOD plus||10x buffer||dNTP||MgSO4||F primer||R primer||KOD-plus||MilliQ||total | ||
| + | |- | ||
| + | |Pcon antisense(1C3-GGE-fwd,1A2-GG2-rev)||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |- | ||
| + | |DT-(1C3-GG1-fwd,1C3-GG2-rev)||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |- | ||
| + | |Spinach-DT-(1C3-GG1-fwd,1C3-GG2-rev)||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |- | ||
| + | |Pcon-antisense-spinach-DT(1C3-GGE-fwd,1A2-GG1-rev) ||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |- | ||
| + | |Ptet(1C3-GGE-fwd,1C3-GG0-rev) ||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |- | ||
| + | |Pcon attenuator(1C3-GGE-fwd,1C3-GG0-rev) ||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |- | ||
| + | |DT(1C3-GG2-fwd,1C3-GG1-rev)||1µL||2.5µL||2.5µL||1.5µL||0.75µL||0.75µL||0.5µL||15.5||25µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||98°C||57°C||68°C||-- | ||
| + | |- | ||
| + | |2min||10sec||30sec||36sec||30 | ||
| + | |} | ||
| + | </div> | ||
Latest revision as of 18:22, 27 September 2013
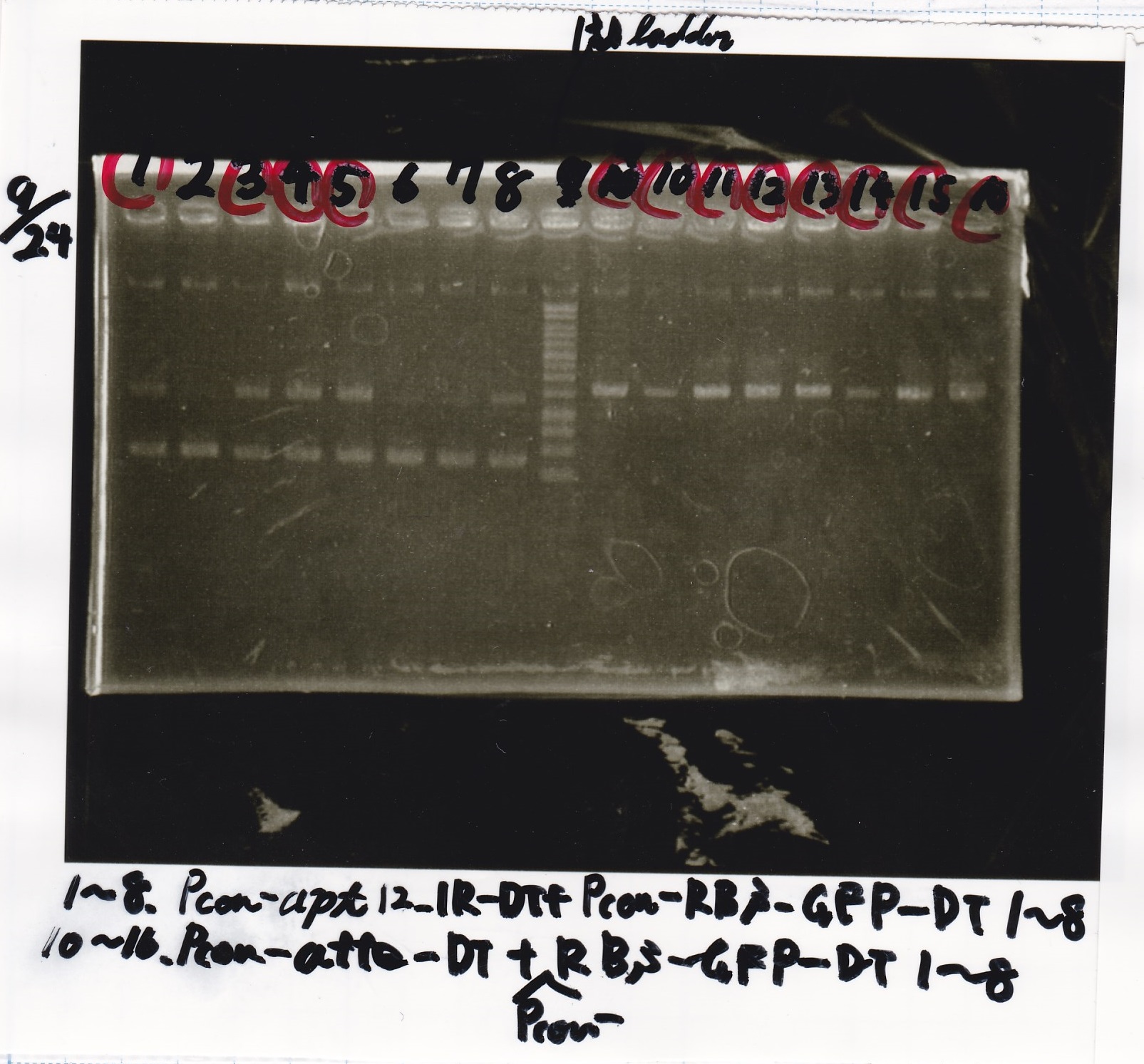
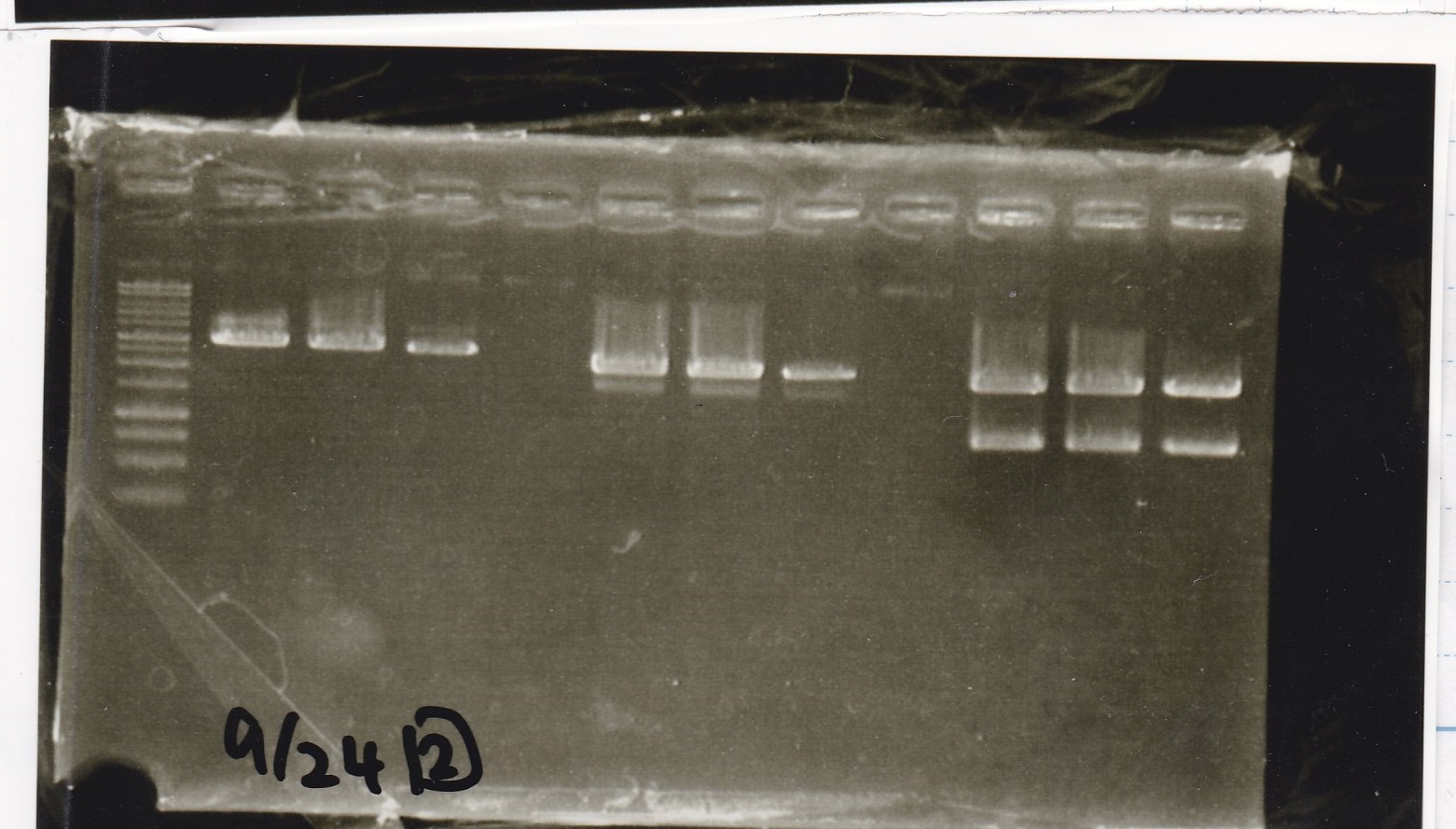
Sep 24
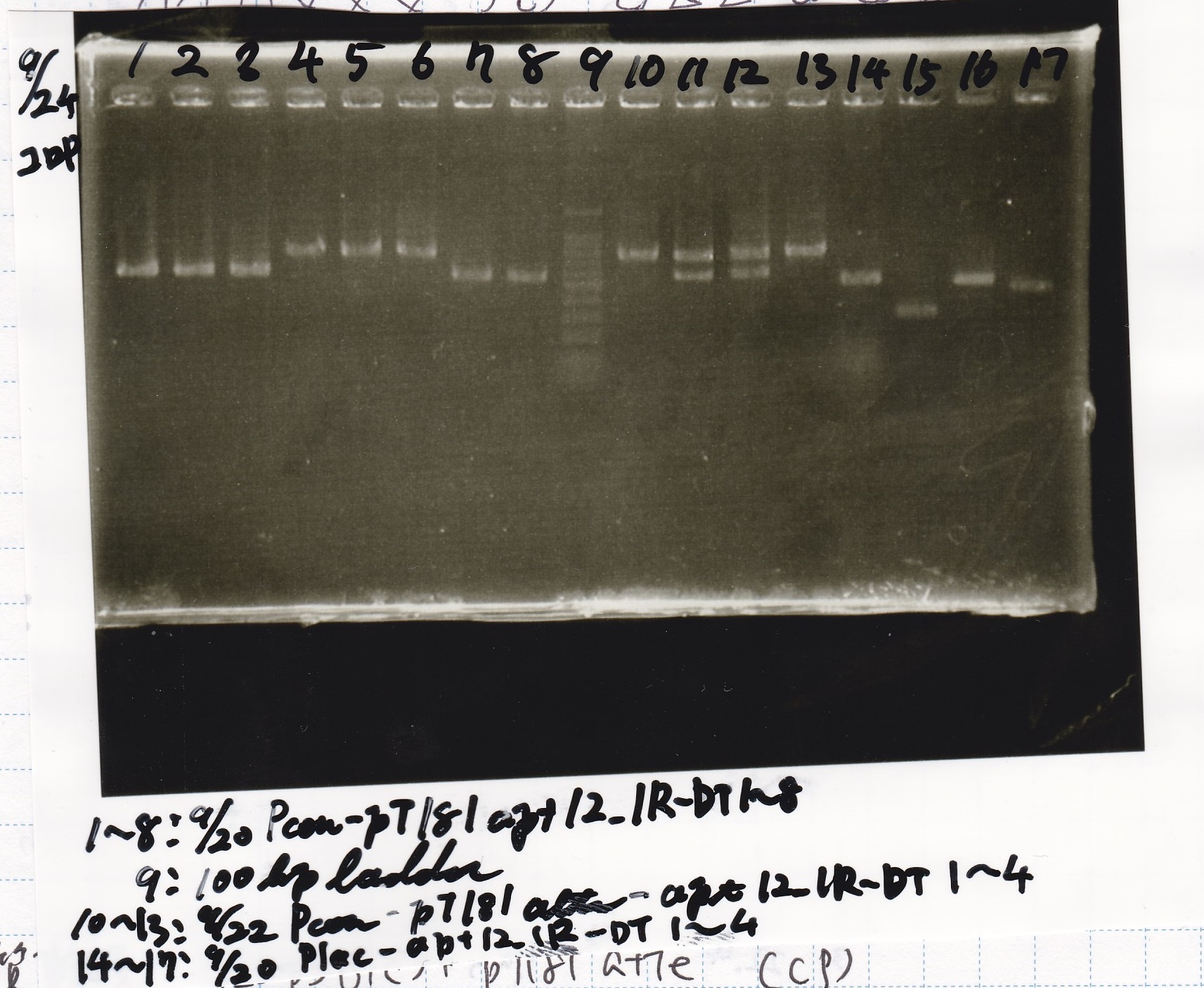
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 9/20 Pcon-pT181aptamer12-1R-DT 1 |
| 2 | 9/20 Pcon-pT181aptamer12-1R-DT 2 |
| 3 | 9/20 Pcon-pT181aptamer12-1R-DT 3 |
| 4 | 9/20 Pcon-pT181aptamer12-1R-DT 4 |
| 5 | 9/20 Pcon-pT181aptamer12-1R-DT 5 |
| 6 | 9/20 Pcon-pT181aptamer12-1R-DT 6 |
| 7 | 9/20 Pcon-pT181aptamer12-1R-DT 7 |
| 8 | 9/20 Pcon-pT181aptamer12-1R-DT 8 |
| 9 | 100bp ladder |
| 10 | 9/22 Pcon-pT181attenuator-aptamer12-1R-DT 1 |
| 11 | 9/22 Pcon-pT181attenuator-aptamer12-1R-DT 2 |
| 12 | 9/22 Pcon-pT181attenuator-aptamer12-1R-DT 3 |
| 13 | 9/22 Pcon-pT181attenuator-aptamer12-1R-DT 4 |
| 14 | 9/20 Plac-aptamer12-1R-DT 1 |
| 15 | 9/20 Plac-aptamer12-1R-DT 2 |
| 16 | 9/20 Plac-aptamer12-1R-DT 3 |
| 17 | 9/20 Plac-aptamer12-1R-DT 4 |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| 9/23 pSB1C3-pT181attenuator | 2 µL | 20 µL | 22 µL | LB (+CP) |
| RBS-GFP-DT+Pcon-pT181attenuator | 2 µL | 20 µL | 22 µL | LB (+CP) |
| 9/23 Plac-+spinach-DT | 2 µL | 20 µL | 22 µL | LB (+Amp) |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT | 2 µL | 20 µL | 22 µL | LB (+Amp) |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT | 2 µL | 20 µL | 22 µL | LB (+Amp) |
| 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT | 2 µL | 20 µL | 22 µL | LB (+Amp) |
| 9/23 Pcon-pT181antisense-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
| 9/23 Pcon-pT181attenuator-RBS-GFP-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
| 9/23 Pcon-pT181attenuator-aptamer12-1R-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
| PUC19 | 2 µL | 20 µL | 22 µL | LB (+Amp) |
| Ptet-RBS-lacZα-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
| pSB1C3 | 2 µL | 20 µL | 22 µL | LB (+CP) |
Liquid Culture
| Sample | medium |
|---|---|
| aptamer12-1R-DT(Master plate) | LB(+CP) |
Restriction Enzyme Digestion(PCR product)
Concentrate PCR product by evaporator until volume become less than 1µL, add all sulution and pipetting.
| 10*cut smart buffer | BsaI-HF | MilliQ | total | |
|---|---|---|---|---|
| 9/22 Pcon-pT181 attenuator (E-0) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-pT181 attenuator (2-3A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 spinach-DT(3-2) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Ptet-pT181 antisense (1-3) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-tet aptamer-DT (E-1A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-spinach-DT (E-1A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-pT181 attenuator (E-1A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 DT (1-3) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 tet aptamer-DT (1-3) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-pT181 attenuator (1-SA) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-RBS-tetR-DT (1-2A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-RBS-tetR-DT (3-2A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-RBS-tetR-DT (E-2A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-RBS-GFP-DT (1-2A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/22 Pcon-RBS-GFP-DT (2-SA) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/23 Pcon-pT181 attenuator-DT (E-1A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/23 Pcon-pT181 attenuator-DT (0-1A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/23 Pcon-pT181 antisense-spinach-DT (1-2A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/23 Pcon-pT181 antisense (1-2A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/23 Ptet-1 (2-S) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
| 9/23 Plac-2 (E-1A) | 2.0 µL | 0.3 µL | 17 µL | 19.3µL |
Incubate 37°C 12hour</div>
Column Refining
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/22 Pcon-pT181 attenuator(E-0) | 7.8 | 1.70 | 0.03 |
| 8/22 Pcon-pT181 attenuator(2-SA) | 17.3 | 1.93 | 0.03 |
| 8/22 spinach-DT (3-2) | 22.4 | 1.93 | 0.09 |
| 8/22 Ptet-PT181 anisense (1-3) | 18.3 | 1.58 | 0.14 |
| 8/22 Pcon-aptamer12_1R-DT (E-1A) | 8.2 | 2.10 | 0.05 |
| 8/22 Pcon-spinach-DT(E-1A) | 5.6 | 0.91 | 0.05 |
| 8/22 Pcon-pT181 attenuator(E-1A) | 18.5 | 1.80 | 0.12 |
| 8/22 DT(1-3) | 7.3 | 1.13 | 0.04 |
| 8/22 tetR aptamer 12_1R-DT(1-3) | 14.6 | 1.74 | 0.06 |
| 8/22 Pcon-pT181 attenuator(1-SA) | 7.9 | 1.57 | 0.04 |
| 8/22 Pcon-RBS-tetR-DT(1-2Ⓐ) | 41.6 | 1.81 | 0.25 |
| 8/22 Pcon-RBS-tetR-DT(3-2Ⓐ) | 30.2 | 1.75 | 0.15 |
| 8/22 Pcon-RBS-tetR-DT(E-2Ⓐ) | 36.9 | 1.75 | 0.56 |
| 8/22 Pcon-RBS-GFP-DT(1-SⒶ) | 43.5 | 1.83 | 0.33 |
| 8/22 Pcon-RBS-GFP-DT(2-SⒶ) | 64.6 | 1.82 | 0.30 |
| 8/23 Pcon-pT181 attenuator-DT (E-1A) | 31.2 | 1.83 | 0.09 |
| 8/23 Pcon-pT181 attenuator-DT (0-1A) | 64.5 | 1.80 | 0.43 |
| 8/23 Pcon-pT181 antisense-spinach-DT (1-2A) | 36.4 | 1.88 | 0.21 |
| 8/23 Pcon-pT181 antisense-spinach-DT (E-1A) | 27.9 | 1.92 | 0.16 |
| 8/23 Ptet(1)(2-S) | 37.0 | 1.91 | 0.16 |
| 9/23 Plac-2(E-1A) | 21.2 | 1.82 | 0.13 |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| 9/23 Pcon-attenuator-aptamer12-1R-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
| 9/23 pT181attenuator(1C3) | 2 µL | 20 µL | 22 µL | LB (+CP) |
| 9/23 Pcon-pT181antisense-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
| 9/23 Pcon-pT181attenuator-RBS--GFP-DT | 2 µL | 20 µL | 22 µL | LB (+CP) |
Liquid Culture
| Sample | medium |
|---|---|
| pSB1C3 (Master plate-1) | LB(+CP) |
Restriction Enzyme Digestion
| 8/21 pT181attenuator (330 µg/mL) | XbaI | PstI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 6.1 | 1 | 1 | 3 | 3 | 15.9 | 30 |
| NC | 0.3 | 0 | 0 | 1 | 1 | 7.7 | 10 |
| 9/22 Pcon-pT181attenuator (368.3 µg/mL) | EcoRI | SpeI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 5.4 | 1 | 1 | 3 | 0 | 19.6 | 30 |
| NC | 0.3 | 0 | 0 | 1 | 0 | 8.7 | 10 |
| 9/16 pSB1C3 | EcoRI | SpeI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2 cuts | 18 | 1 | 1 | 3 | 0 | 7.0 | 30 |
| NC | 0.9 | 0 | 0 | 1 | 0 | 8.1 | 10 |
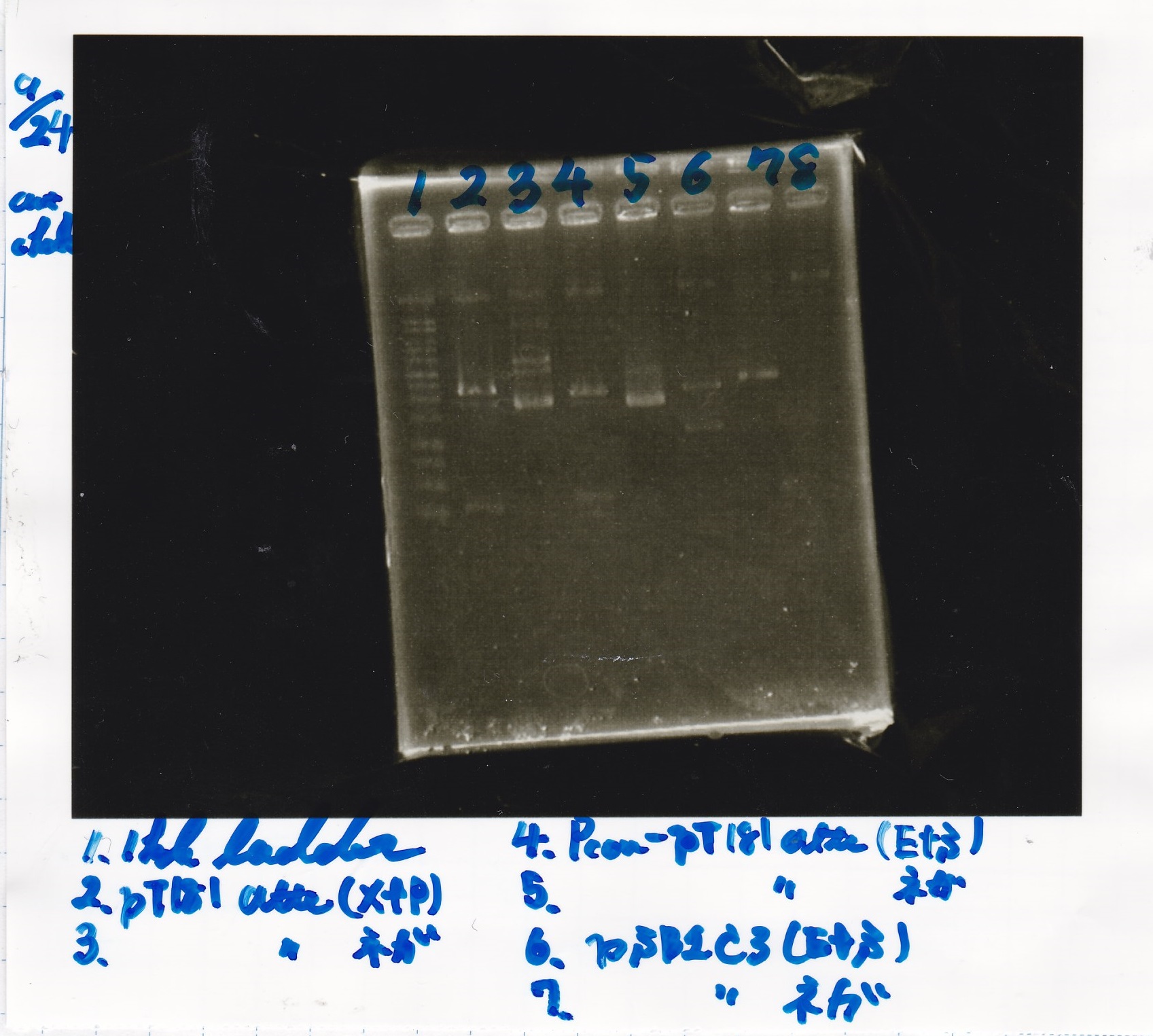
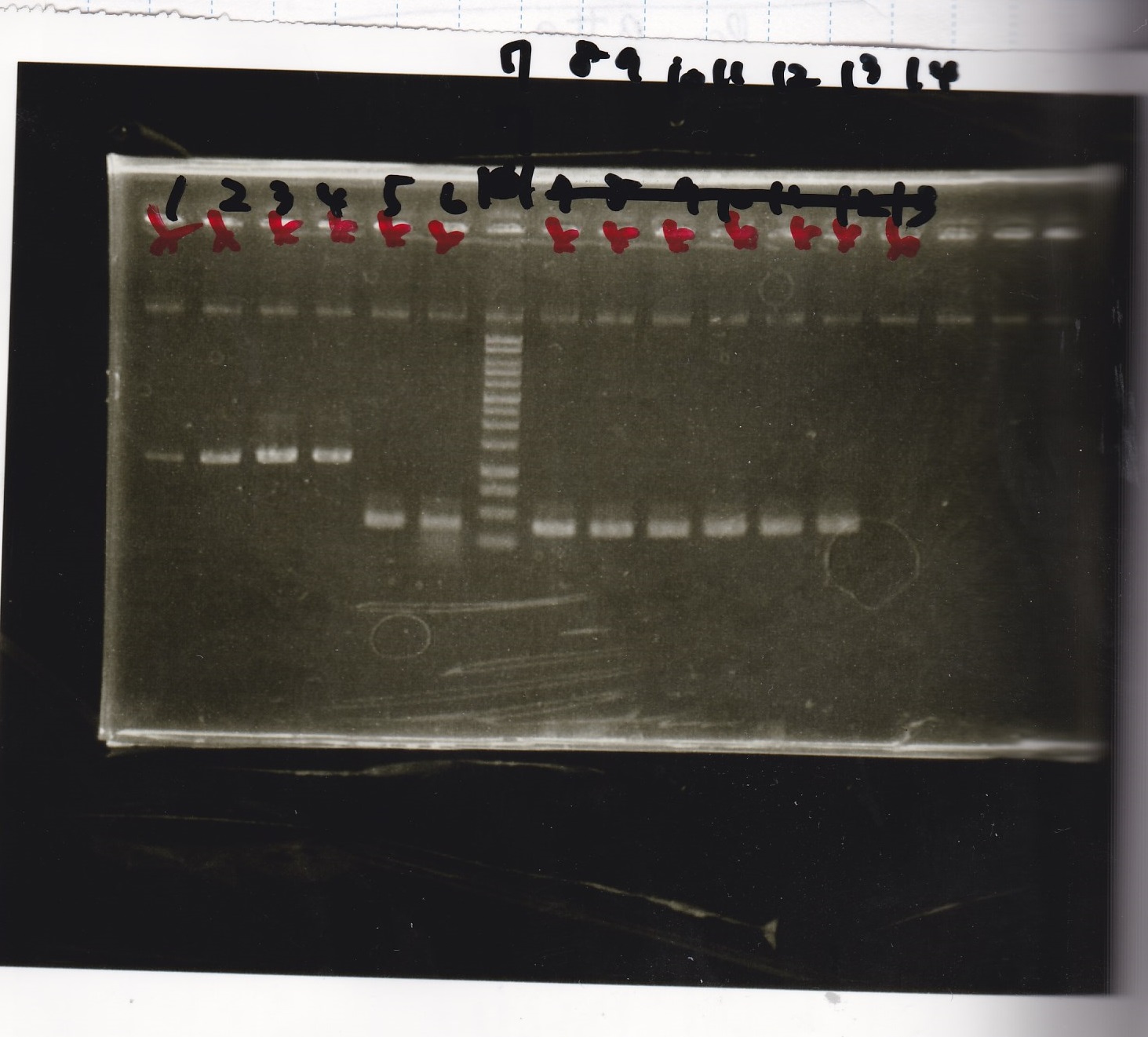
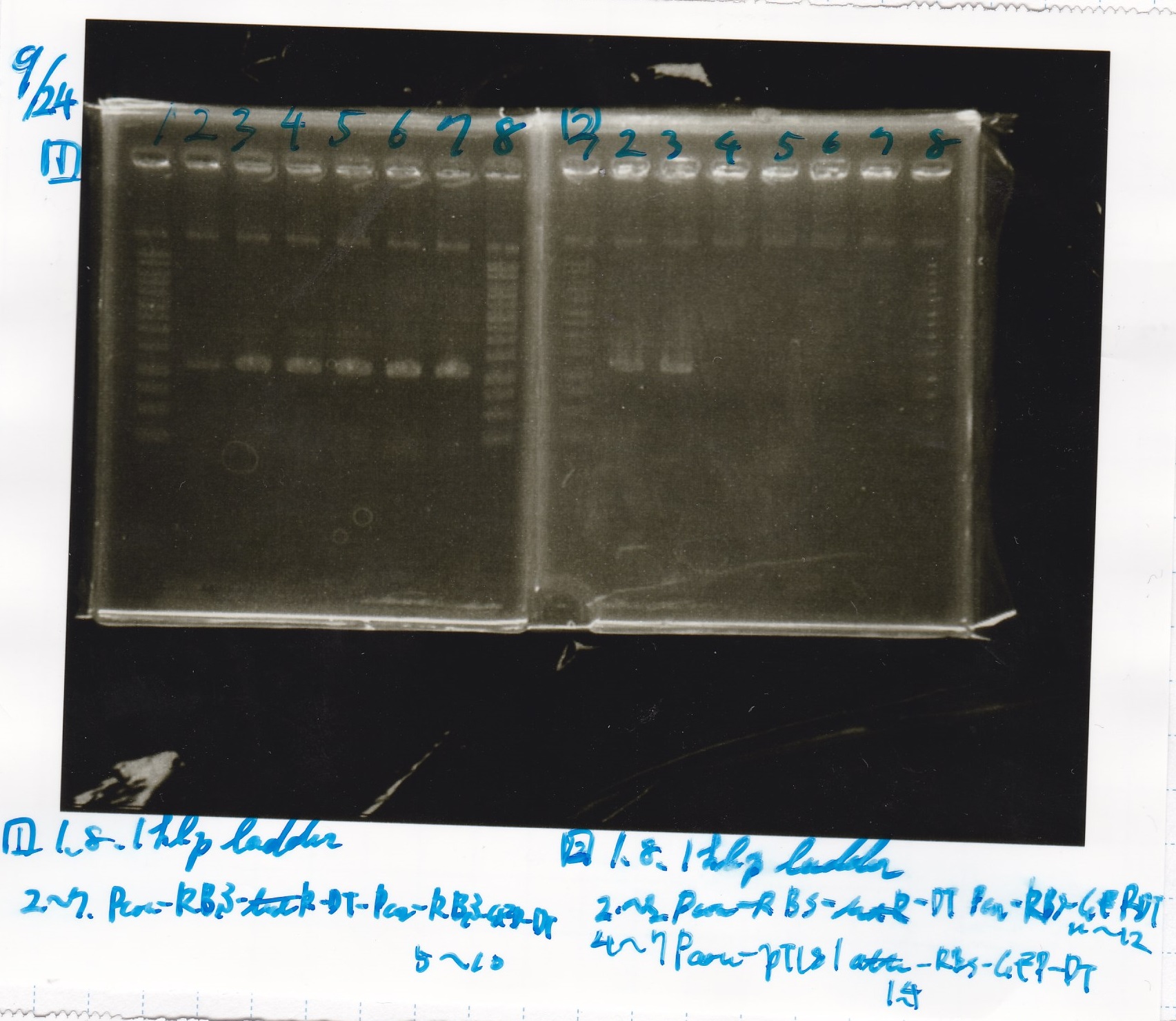
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kb ladder | - | - |
| 2 | pT181attenuator | XbaI | PstI |
| 3 | pT181attenuator | -- | -- |
| 4 | Pcon-pT181attenuator | EcoRI | SpeI |
| 5 | Pcon-pT181attenuator | -- | -- |
| 6 | pSB1C3 | EcoRI | SpeI |
| 7 | pSB1C3 | -- | -- |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/24 aptamer12-1R-DT | 144.7 | 1.03 | 1.21 |
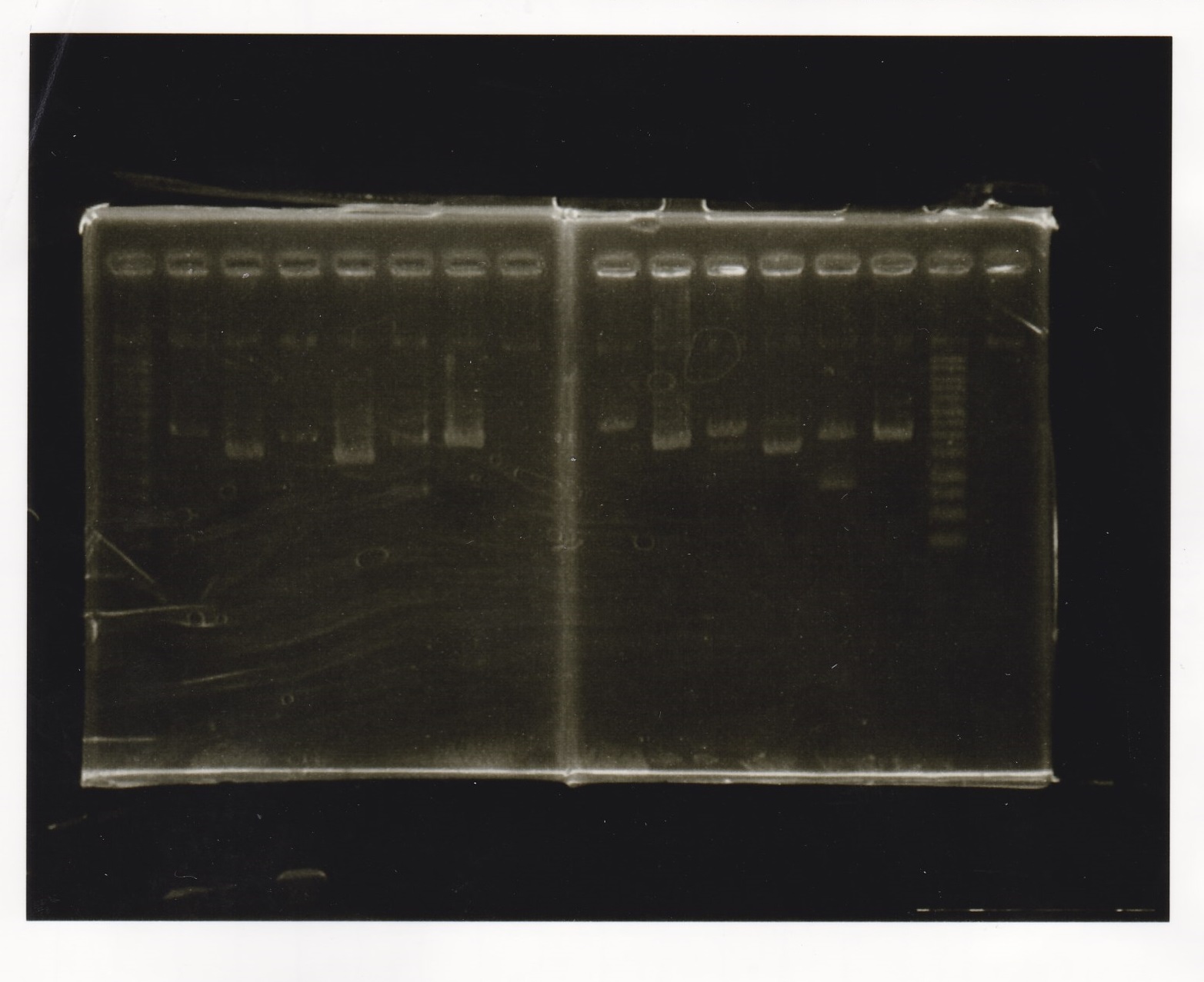
Gel Extraction
| Lane | DNA | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100kb ladder | -- | -- |
| 2 | pT181attenuator | XbaI | PstI |
| 3 | pT181attenuator | XbaI | PstI |
| 4 | pT181attenuator | XbaI | PstI |
| 5 | -- | -- | -- |
| 6 | Pcon-pT181attenuator | EcoRI | SpeI |
| 7 | Pcon-pT181attenuator | EcoRI | SpeI |
| 8 | Pcon-pT181attenuator | EcoRI | SpeI |
| 9 | -- | -- | -- |
| 10 | pSB1C3 | EcoRI | SpeI |
| 11 | pSB1C3 | EcoRI | SpeI |
| 12 | pSB1C3 | EcoRI | SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pT181attenuator(XbaI&PstI) | 10.1 | 2.02 | 0.43 |
| Pcon-pT181attenuator(EcoRI&SpeI) | 10.3 | 1.81 | 0.32 |
| pSB1C3(EcoRI&SpeI) | 10.0 | 2.81 | 0.02 |
Colony PCR
| Sample | base pair |
|---|---|
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 1 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 2 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 3 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 4 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 5 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 6 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 7 | 1414 |
| 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 8 | 1414 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 1 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 2 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 3 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 4 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 5 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 6 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 7 | 1631 |
| 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 8 | 1631 |
| 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 1 | 2042 |
| 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 2 | 2042 |
| 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 3 | 2042 |
| 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 4 | 2042 |
| 9/23 Pcon-pT181antisense-DT 1 | 508 |
| 9/23 Pcon-pT181antisense-DT 2 | 508 |
| 9/23 Pcon-pT181antisense-DT 3 | 508 |
| 9/23 Pcon-pT181antisense-DT 4 | 508 |
| 9/23 Pcon-pT181antisense-DT 5 | 508 |
| 9/23 Pcon-pT181antisense-DT 6 | 508 |
| 9/23 Pcon-pT181antisense-DT 7 | 508 |
| 9/23 Pcon-pT181antisense-DT 8 | 508 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94 °C | 94 °C | 55 °C | 68 °C | -- |
| 5 min | 30 sec | 30 sec | 2min | 30 cycles |
| Lane | Sample |
|---|---|
| 1 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 1 |
| 2 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 2 |
| 3 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 3 |
| 4 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 4 |
| 5 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 5 |
| 6 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 6 |
| 7 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 7 |
| 8 | 9/23 Pcon-aptamer12-1R-DT+Pcon-RBS-GFP-DT 8 |
| 9 | 1kb ladder |
| 10 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 1 |
| 11 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 2 |
| 12 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 3 |
| 13 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 4 |
| 14 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 5 |
| 15 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 6 |
| 16 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 7 |
| 17 | 9/23 Pcon-pT181attenuator-DT+Pcon-RBS-GFP-DT 8 |
| Lane | Sample |
|---|---|
| 1 | 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 1 |
| 2 | 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 2 |
| 3 | 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 3 |
| 4 | 9/23 Pcon-RBS-tetR-DT+Pcon-RBS-GFP-DT 4 |
| 5 | 9/23 Pcon-pT181antisense-DT 1 |
| 6 | 9/23 Pcon-pT181antisense-DT 2 |
| 7 | 1kb ladder |
| 8 | 9/23 Pcon-pT181antisense-DT 3 |
| 9 | 9/23 Pcon-pT181antisense-DT 4 |
| 10 | 9/23 Pcon-pT181antisense-DT 5 |
| 11 | 9/23 Pcon-pT181antisense-DT 6 |
| 12 | 9/23 Pcon-pT181antisense-DT 7 |
| 13 | 9/23 Pcon-pT181antisense-DT 8 |
| 14 | -- |
Restriction Enzyme Digestion
| 9/24 aptamer12-1R-DT | EcoRI | XbaI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 17.5µL | 1.0µL | 1.0µL | 3.0µL | 3.0µL | 4.5µL | 30µL |
| NC | 0.9µL | 0µL | 0µL | 1.0µL | 1.0µL | 7.1µL | 10µL |
| 9/20 Ptet | SpeI | PstI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 9.5µL | 1.0µL | 1.0µL | 3.0µL | 0µL | 15.5µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1.0µL | 0µL | 8.5µL | 10µL |
| 9/22 RBS-GFP-DT | EcoRI | SpeI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 3.8µL | 1.0µL | 1.0µL | 3.0µL | 0µL | 21.2µL | 30µL |
| NC | 0.2µL | 0µL | 0µL | 1.0µL | 0µL | 8.8µL | 10µL |
| 9/22 Pcon-RBS-GFP-DT | EcoRI | XbaI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 2.4µL | 1.0µL | 1.0µL | 3.0µL | 3.0µL | 19.6µL | 30µL |
| NC | 0.1µL | 0µL | 0µL | 1.0µL | 1.0µL | 7.9µL | 10µL |
| 9/22 Pcon-pT181attenuator 1 | EcoRI | XbaI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 5.4µL | 1.0µL | 1.0µL | 3.0µL | 3.0µL | 16.6µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 1.0µL | 1.0µL | 7.7µL | 10µL |
| 9/22 RBS-GFP-DT | XbaI | PstI | buffer | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 3.8µL | 1.0µL | 1.0µL | 3.0µL | 3.0µL | 18.2µL | 30µL |
| NC | 0.2µL | 0µL | 0µL | 1.0µL | 1.0µL | 7.8µL | 10µL |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/22 aptamer12-1R-DT(EcoRI&XbaI) | 1.4 µL | 9/24 Pcon-pT181attenuator(EcoRI&SpeI) | 26 µL | 3.5 µL |
| experiment | 9/4 Ptet(SpeI&PstI) | 2.0 µL | 9/21 RBS-GFP-DT(XbaI&PstI) | 9.0 µL | 3.5 µL |
- incubated at 37 °C for 1 hour.
colony PCR
| Sample | base pair |
|---|---|
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 5 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 6 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 7 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 8 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 9 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 10 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 11 | 2042bp |
| 9/23 Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 12 | 2042bp |
| 9/23 Pcon-pT181antisense-DT 9 | 508bp |
| 9/23 Pcon-pT181antisense-DT 10 | 508bp |
| 9/23 Pcon-pT181antisense-DT 11 | 508bp |
| 9/23 Pcon-pT181antisense-DT 12 | 508bp |
| 9/23 Pcon-pT181antisense-DT 13 | 508bp |
| 9/24 Pcon-pT181attenuator-RBS-GFP-DT 1 | 1527bp |
| 9/24 Pcon-pT181attenuator-RBS-GFP-DT 2 | 1527bp |
| 9/24 Pcon-pT181attenuator-RBS-GFP-DT 3 | 1527bp |
| 9/24 Pcon-pT181attenuator-RBS-GFP-DT 4 | 1527bp |
| 9/24 Pcon-pT181attenuator-aptamer12-1R-DT 1 | 859bp |
| 9/24 Pcon-pT181attenuator-aptamer12-1R-DT 2 | 859bp |
| 9/24 Pcon-pT181attenuator-aptamer12-1R-DT 3 | 859bp |
| 9/24 Pcon-pT181attenuator-aptamer12-1R-DT 4 | 859bp |
| 9/24 pT181attenuator(pSB1C3) 1 | 390bp |
| 9/24 pT181attenuator(pSB1C3) 2 | 390bp |
| 9/24 pT181attenuator(pSB1C3) 3 | 390bp |
| 9/24 pT181attenuator(pSB1C3) 4 | 390bp |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 2min | 30cycle |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| pSB1C3 | 183.9 | 1.91 | 1.59 |
| Pcon-pT181attenuator-aptamer12-1R-DT | 231.7 | 1.82 | 2.35 |
| Plac-aptamer12-1R-DT | 208.9 | 1.98 | 1.79 |
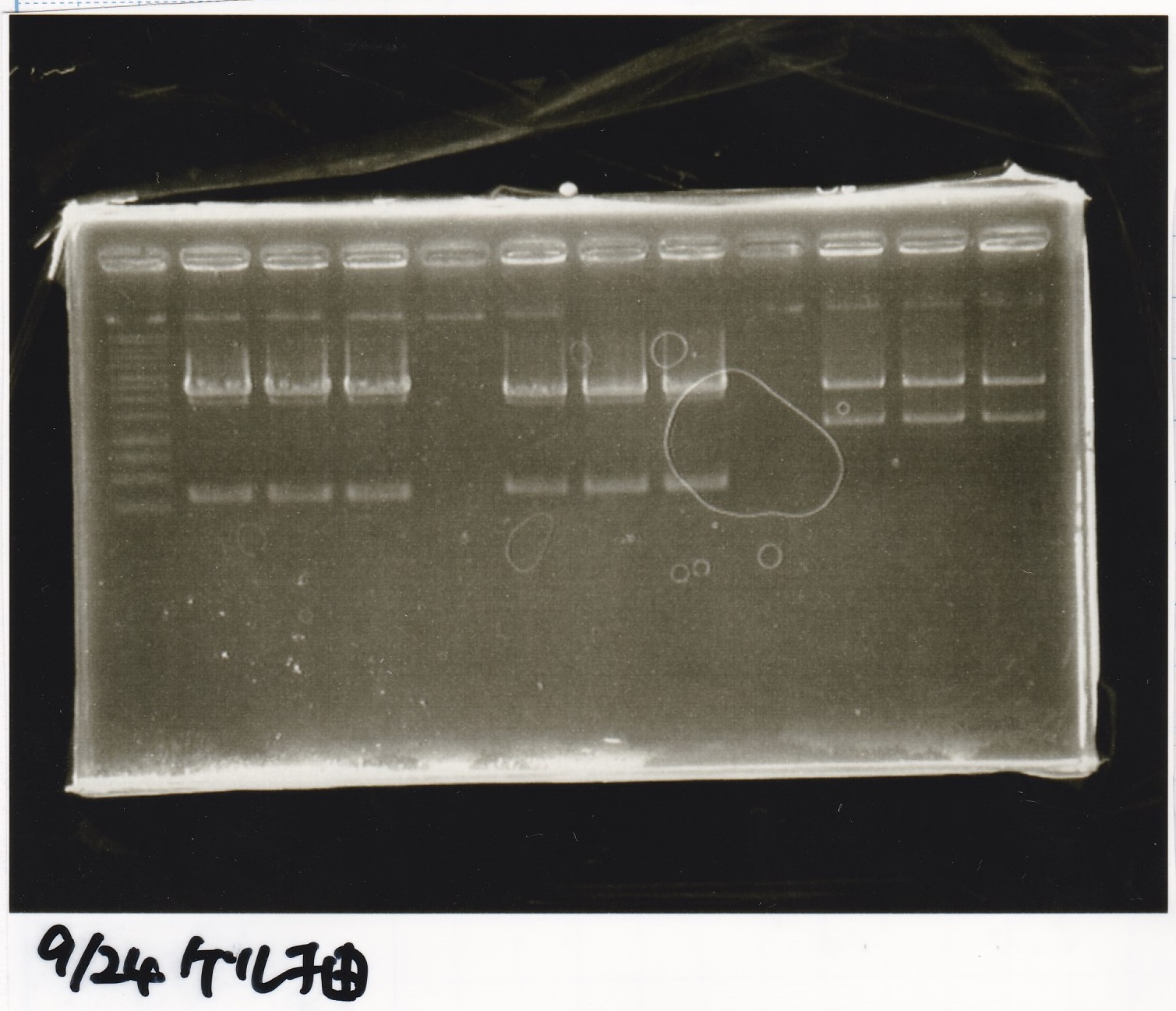
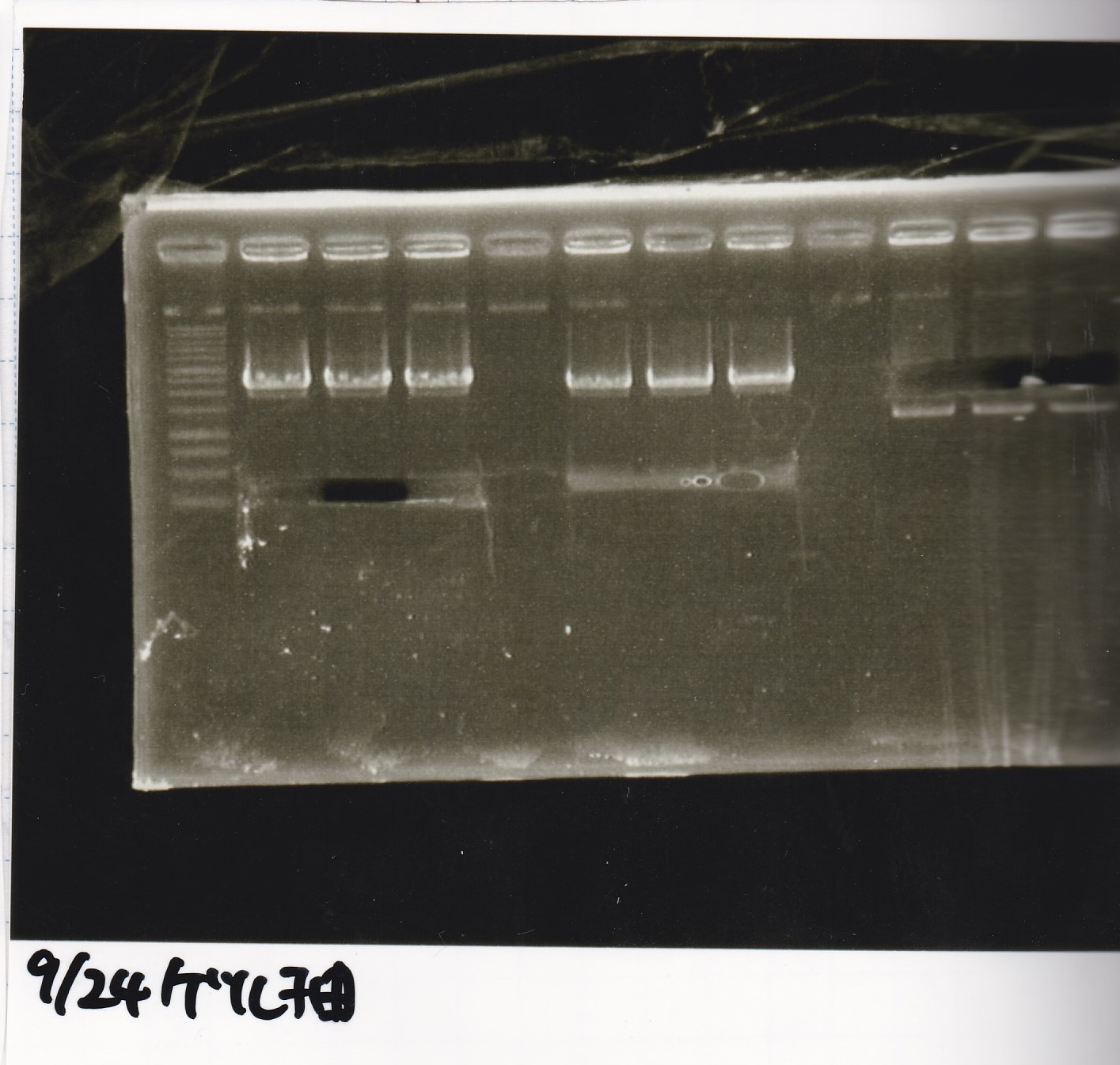
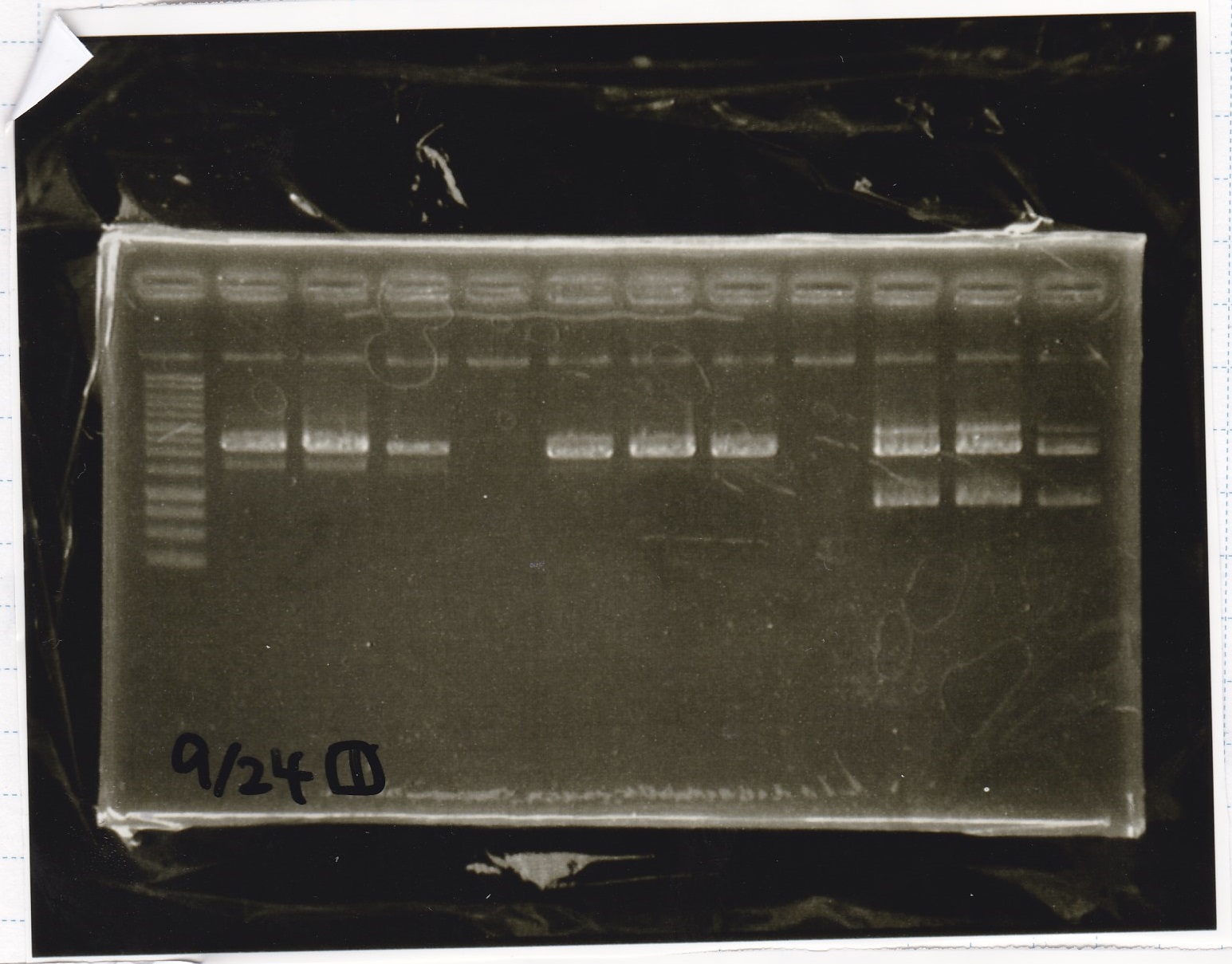
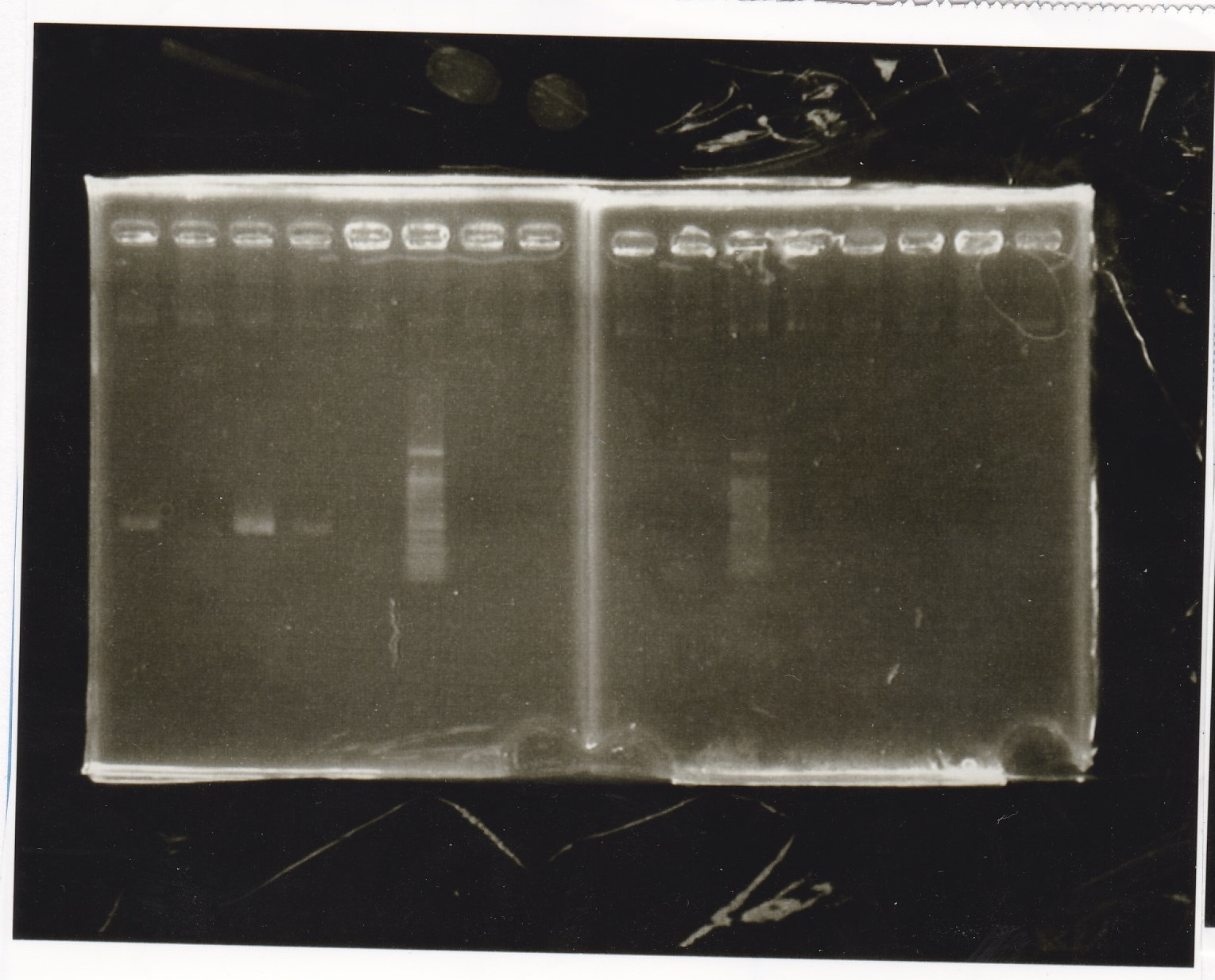
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kb ladder | -- | -- |
| 2 | 9/24 aptamer12-1R-DT | EcoRI | XbaI |
| 3 | 9/24 aptamer12-1R-DT | -- | -- |
| 4 | 9/24 Ptet | SpeI | PstI |
| 5 | 9/24 Ptet | -- | -- |
| 6 | 9/24 RBS-GFP-DT | EcoRI | SpeI |
| 7 | 9/24 RBS-GFP-DT | -- | -- |
| 8 | 9/24 Pcon-RBS-GFP-DT | EcoRI | XbaI |
| 8 | 9/24 Pcon-RBS-GFP-DT | -- | -- |
| 9 | 9/24 Pcon-RBS-GFP-DT | -- | -- |
| 10 | 9/24 Pcon-pT181attenuator 1 | EcoRI | XbaI |
| 11 | 9/24 Pcon-pT181attenuator 1 | -- | -- |
| 12 | 9/24 RBS-GFP-DT | XbaI | PstI |
| 13 | 9/24 RBS-GFP-DT | -- | -- |
| 14 | 1kb ladder | -- | -- |
Gel Extraction
1
| Lane | DNA | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kb ladder | -- | -- |
| 2 | aptamer12-1R-DT | EcoRI | XbaI |
| 3 | aptamer12-1R-DT | EcoRI | XbaI |
| 4 | aptamer12-1R-DT | EcoRI | XbaI |
| 5 | -- | -- | -- |
| 6 | Ptet | SpeI | PstI |
| 7 | Ptet | SpeI | PstI |
| 8 | Ptet | SpeI | PstI |
| 9 | -- | -- | -- |
| 10 | RBS-GFP-DT | EcoRI | SpeI |
| 11 | RBS-GFP-DT | EcoRI | SpeI |
| 12 | RBS-GFP-DT | EcoRI | SpeI |
| Lane | DNA | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kb ladder | -- | -- |
| 2 | Pcon-RBS-GFP-DT | EcoRI | XbaI |
| 3 | Pcon-RBS-GFP-DT | EcoRI | XbaI |
| 4 | Pcon-RBS-GFP-DT | EcoRI | XbaI |
| 5 | -- | -- | -- |
| 6 | Pcon-pT181attenuator | EcoRI | XbaI |
| 7 | Pcon-pT181attenuator | EcoRI | XbaI |
| 8 | Pcon-pT181attenuator | EcoRI | XbaI |
| 9 | -- | -- | -- |
| 10 | RBS-GFP-DT | XbaI | PstI |
| 11 | RBS-GFP-DT | XbaI | PstI |
| 12 | RBS-GFP-DT | XbaI | PstI |
Electrophoresis
1
| Lane | Sample |
|---|---|
| 1 | 1kb ladder |
| 2 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 5 |
| 3 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 6 |
| 4 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 7 |
| 5 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 8 |
| 6 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 9 |
| 7 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 10 |
| 8 | 1kb ladder |
2
| Lane | Sample |
|---|---|
| 1 | 1kb ladder |
| 2 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 11 |
| 3 | Pcon-RBS-tetR-DT-Pcon-RBS-GFP-DT 12 |
| 4 | Pcon-pT181attenuator-RBS-GFP-DT 1 |
| 5 | Pcon-pT181attenuator-RBS-GFP-DT 2 |
| 6 | Pcon-pT181attenuator-RBS-GFP-DT 3 |
| 7 | Pcon-pT181attenuator-RBS-GFP-DT 4 |
| 8 | 1kb ladder |
3
| Lane | Sample |
|---|---|
| 1 | Pcon-pT181antisense-DT 9 |
| 2 | Pcon-pT181antisense-DT 10 |
| 3 | Pcon-pT181antisense-DT 11 |
| 4 | Pcon-pT181antisense-DT 12 |
| 5 | Pcon-pT181antisense-DT 13 |
| 6 | 100bp ladder |
| 7 | Pcon-pT181attenuator-aptamer12-1R-DT 1 |
| 8 | Pcon-pT181attenuator-aptamer12-1R-DT 2 |
4
| Lane | Sample |
|---|---|
| 1 | Pcon-pT181attenuator-aptamer12-1R-DT 3 |
| 2 | Pcon-pT181attenuator-aptamer12-1R-DT 4 |
| 3 | 100bp ladder |
| 4 | pT181attenuator(pSB1C3) 1 |
| 5 | pT181attenuator(pSB1C3) 2 |
| 6 | pT181attenuator(pSB1C3) 3 |
| 7 | pT181attenuator(pSB1C3) 4 |
Ligation
| state | Vector | Inserter1 | Inserter2 | Inserter3 | Inserter4 | (NEB)T4ligase | (NEB)Buffer | milliQ | total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | tetR-aptamer12-1R-DT(1-3) | 1 µL | Ptet(2-S) | 0.3µL | Pcon-tetR-aptamer12-1R-DT(3-2A) | 1.1µL | Plac(E-1A) | 0.5µL | 0.5µL | 0.5µL | 0.2 µL | 5µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-pT181attenuator(E-1A) | 1 µL | Pcon-tetR-aptamer12-1R-DT(1-2A) | 1µL | Ptet(2-S) | 0.3µL | -- | -- | 0.5µL | 0.5µL | 0.7 µL | 5µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-tetRaptamer12-1R-DT(E-1A) | 2µL | Pcon-tetRaptamer12-1R-DT(1-2A) | 1µL | Ptet(2-S) | 0.3µL | -- | -- | 0.5µL | 0.5µL | 0 µL | 5.3µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-spinach-DT(E-1A) | 3µL | Pcon-tetRaptamer12-1R-DT(1-2A) | 1µL | Ptet(2-S) | 0.3µL | -- | -- | 0.5µL | 0.5µL | 0 µL | 6.3µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-pT181antisense-DT(E-1A) | 0.5µL | DT(1-3) | 2µL | Ptet(2-S) | 0.3µL | Pcon-tetRaptamer12-1R-DT(3-2A) | 1.1µL | 0.5µL | 0.5µL | 0.1 µL | 5µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-pT181attenuator(E-1A) | 1µL | tetRaptamer12-1R-DT(1-3) | 1µL | Ptet(2-S) | 0.3µL | Pcon-tetRaptamer12-1R-DT(3-2A) | 1.1µL | 0.5µL | 0.5µL | 0µL | 5.4µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Ptet(2-S) | 0.3µL | Pcon-tetRaptamer12-1R-DT(E-2A) | 1µL | -- | -- | -- | -- | 0.5µL | 0.5µL | 1.7µL | 5µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-GFP-DT(2-SA) | 0.5µL | 1µL | -- | -- | -- | -- | -- | 0.5µL | 0.5µL | 1.5µL | 5µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-tetRaptamer12-1R-DT(E-1A) | 2µL | Pcon-pT181attenuator(1-SA) | 2.1µL | -- | -- | -- | -- | 0.5µL | 0.5µL | 0µL | 6.1µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-pT181attenuator-DT(E-1A) | 1µL | Pcon-pT181attenuator(1-SA) | 2.1µL | -- | -- | -- | -- | 0.5µL | 0.5µL | 0µL | 5.1µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-tetR12-1R-DT(E-1A) | 2µL | Pcon-GFP-DT(2-SA) | 0.5µL | spinach-DT(3-2) | 1µL | Ptet-pT181antisense(1-3) | 1µL | 0.5µL | 0.5µL | 0µL | 6.5µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-tetRaptamer12-1R-DT(E-1A) | 2µL | Pcon-pT181antisense(1-2A) | 0.7µL | Pcon-pT181attenuator(2-SA) | 2.1µL | -- | -- | 0.5µL | 0.5µL | 0µL | 6.8µL |
| experiment | RBS-GFP-DT(pSB1C3) | 1µL | Pcon-tetRaptamer12-1R-DT(E-1A) | 2µL | Pcon-pT181attenuator(2-SA) | 2.1µL | Spinach-DT(3-2) | 1µL | Ptet-pT181antisense(1-3) | 1µL | 0.5µL | 0.5µL | 0µL | 8.1µL |
Concentrate total (more than 5µL) by evaporater until volume become less than 5 µL
PCR
| genome DNA | KOD plus | 10x buffer | dNTP | MgSO4 | F primer | R primer | KOD-plus | MilliQ | total |
|---|---|---|---|---|---|---|---|---|---|
| Pcon antisense(1C3-GGE-fwd,1A2-GG2-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| DT-(1C3-GG1-fwd,1C3-GG2-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| Spinach-DT-(1C3-GG1-fwd,1C3-GG2-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| Pcon-antisense-spinach-DT(1C3-GGE-fwd,1A2-GG1-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| Ptet(1C3-GGE-fwd,1C3-GG0-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| Pcon attenuator(1C3-GGE-fwd,1C3-GG0-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| DT(1C3-GG2-fwd,1C3-GG1-rev) | 1µL | 2.5µL | 2.5µL | 1.5µL | 0.75µL | 0.75µL | 0.5µL | 15.5 | 25µL |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 98°C | 57°C | 68°C | -- |
| 2min | 10sec | 30sec | 36sec | 30 |
 "
"