Template:Kyoto/Notebook/Sep 23
From 2013.igem.org
(Difference between revisions)
(→Electrophoresis) |
(→Sep 23) |
||
| (12 intermediate revisions not shown) | |||
| Line 15: | Line 15: | ||
===Gel Extraction=== | ===Gel Extraction=== | ||
| - | + | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No Name</span> | <span class="author">No Name</span> | ||
| Line 30: | Line 30: | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |Pcon-PT181 anti | + | |Pcon-PT181 anti EcoRI+SpeI||46.7 ||1.06 ||0.93 |
|- | |- | ||
| - | |Pcon-RBS-tetR-DT | + | |Pcon-RBS-tetR-DT EcoRI+XbaI||119.3 ||0.99 || |
|- | |- | ||
| - | |RBS-8FP-DT | + | |RBS-8FP-DT EcoRI+XbaI||87.7 ||1.00 ||0.98 |
|- | |- | ||
| - | |ap42_1R-DT | + | |ap42_1R-DT EcoRI+XbaI||68.8 ||1.32 ||1.03 |
|- | |- | ||
|Pcon-RBS-tetR-DT 1-2 A||203.0 ||1.66 ||1.79 | |Pcon-RBS-tetR-DT 1-2 A||203.0 ||1.66 ||1.79 | ||
| Line 49: | Line 49: | ||
|} | |} | ||
</div> | </div> | ||
| - | + | ||
===PCR=== | ===PCR=== | ||
| Line 67: | Line 67: | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | | | + | |94 °C||98 °C||57 °C||68 °C||-- |
|- | |- | ||
| - | | | + | |2min ||10sec ||30sec ||20sec ||30cycles |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
===Ligation=== | ===Ligation=== | ||
| - | + | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Yoshida</span> | <span class="author">Yoshida</span> | ||
| Line 81: | Line 81: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment||9/21 Pcon-RBS-GFP-DT | + | |experiment||9/21 Pcon-RBS-GFP-DT EcoRI+XbaI||47.5 µg/mL||9/15 Pcon-RBS-tetR-DT EcoRI+SpeI||59 µg/mL||2.3 µg/mL |
|- | |- | ||
| - | |experiment||9/21 Pcon-RBS-GFP-DT | + | |experiment||9/21 Pcon-RBS-GFP-DT EcoRI+XbaI||47.5 µg/mL||9/15 Pcon-apt12_1R-DT EcoRI+SpeI||17 µg/mL||2.4 µg/mL |
|- | |- | ||
| - | |experiment||9/21 Pcon-RBS-GFRDT | + | |experiment||9/21 Pcon-RBS-GFRDT EcoRI+XbaI||2.1 µg/mL||9/15 Pcon-DT181 attenuator-DT||21 µg/mL||3.0 µg/mL |
|- | |- | ||
| - | |experiment||9/22 apt 12_1R-DT | + | |experiment||9/22 apt 12_1R-DT EcoRI+XbaI||67.8 µg/mL||9/18 Pcon-DT181 attenuator EcoRI+SpeI||5.7 µg/mL||3.5 µg/mL |
|- | |- | ||
| - | |experiment||9/22RBS-GFP-DT | + | |experiment||9/22RBS-GFP-DT EcoRI+XbaI||87.7 µg/mL||9/18 Pcon-DT18 attenuator EcoRI+SpeI||6.7 µg/mL||3.5 µg/mL |
|- | |- | ||
| - | |experiment||9/13 DT | + | |experiment||9/13 DT EcoRI+XbaI||25.4 µg/mL||9/22 Pcon-DT181 antisense EcoRI+SpeI||46.8 µg/mL||2.3 µg/mL |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
| + | |||
===PCR=== | ===PCR=== | ||
<!-- ここから --> | <!-- ここから --> | ||
| Line 102: | Line 103: | ||
!genome DNA||KOD plus||10x buffer||dNTP||MgSO4||SasA_fwd primer||SasA_rev primer||MilliQ||total | !genome DNA||KOD plus||10x buffer||dNTP||MgSO4||SasA_fwd primer||SasA_rev primer||MilliQ||total | ||
|- | |- | ||
| - | |Pcon- | + | |Pcon-attenuator-DT (E-1A) ||0.5||2.5||2.5||1.5||0.75||0.75||77.5 ||25 |
|- | |- | ||
| - | |Pcon- | + | |Pcon-attenuator-DT (2-1A) ||0.5||2.5||2.5||1.5||0.75||0.75||77.5 ||25 |
|- | |- | ||
| - | |Pcon- | + | |Pcon-antisense-spinach-DT (1-2A) ||0.5||2.5||2.5||1.5||0.75||0.75||77.5 ||25 |
|- | |- | ||
| - | |Pcon- | + | |Pcon-antisense-spinach-DT (E-1A) ||0.5||2.5||2.5||1.5||0.75||0.75||77.5 ||25 |
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
!PreDenature||Denature||Annealing||Extension||cycle | !PreDenature||Denature||Annealing||Extension||cycle | ||
|- | |- | ||
| - | | | + | |94 °C||98 °C||57 °C||68 °C||-- |
|- | |- | ||
| - | | | + | |2min ||10s ||30s ||45s ||30cycle |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
| + | |||
===Miniprep=== | ===Miniprep=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| Line 125: | Line 127: | ||
!DNA||concentration[µg/mL]||260/280||260/230 | !DNA||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |9/22 Plac-DT181 | + | |9/22 Plac-DT181 attenuator-1||204.0 ||2.00 ||1.95 |
|- | |- | ||
| - | |9/22 Plac-DT181 | + | |9/22 Plac-DT181 attenuator-2||195.3 ||1.84 ||1.62 |
|- | |- | ||
| - | |9/22 Plac-DT181 | + | |9/22 Plac-DT181 antisense-1||188.2 ||1.87 ||2.15 |
|- | |- | ||
| - | |9/22 Plac-DT181 | + | |9/22 Plac-DT181 antisense-2||165.0 ||1.95 ||2.66 |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
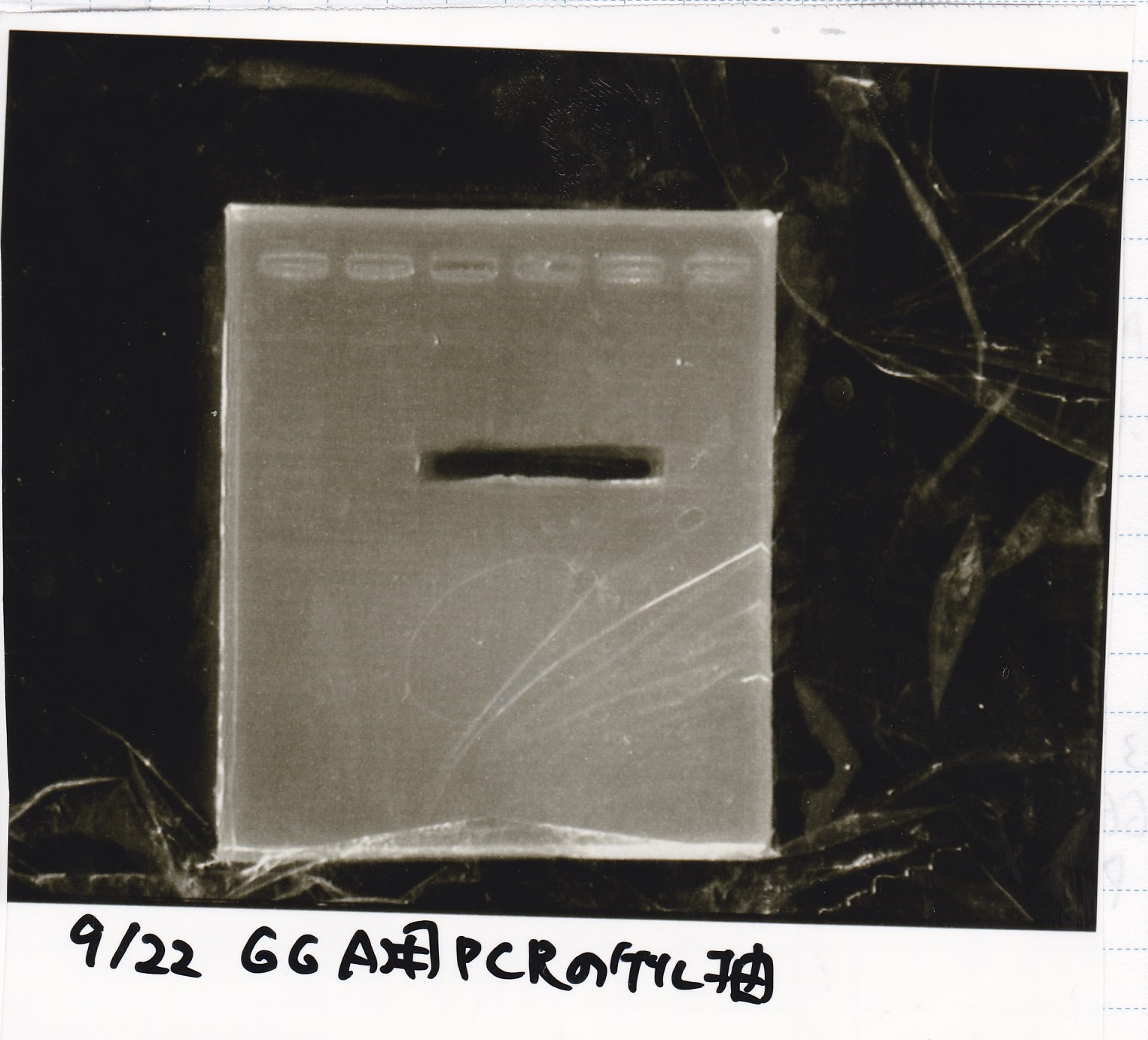
===Electrophoresis=== | ===Electrophoresis=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Hirano</span> | <span class="author">Hirano</span> | ||
| Line 143: | Line 144: | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1,6||100bp ladder(Promega) || || | + | |1,6||100bp ladder(Promega)||--||-- |
|- | |- | ||
| - | |2||Pcon- | + | |2||Pcon-attenuator-DT (E-1A)||--||-- |
|- | |- | ||
| - | |3||Pcon- | + | |3||Pcon-attenuator-DT (2-1A)||--||-- |
|- | |- | ||
| - | |4||Pcon- | + | |4||Pcon-antisense-spinach-DT (1-2A)||--||-- |
|- | |- | ||
| - | |5||Pcon- | + | |5||Pcon-antisense-spinach-DT (E-1A)||--||-- |
|} | |} | ||
[[File:igku_9232.png]]<br> | [[File:igku_9232.png]]<br> | ||
</div> | </div> | ||
| - | |||
===Gel Extraction=== | ===Gel Extraction=== | ||
| - | |||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">Hirano</span> | <span class="author">Hirano</span> | ||
| Line 164: | Line 163: | ||
!Name||concentration[µg/mL]||260/280||260/230 | !Name||concentration[µg/mL]||260/280||260/230 | ||
|- | |- | ||
| - | |Pcon | + | |Pcon-pT181 attenuator-DT(E-1A)||166.7 ||1.51 ||0.99 |
|- | |- | ||
| - | |Pcon | + | |Pcon-pT181 attenuator-DT(2-1A)||122.1 ||1.75 ||1.20 |
|- | |- | ||
| - | |Pcon | + | |Pcon-pT181 antisense-spinach-DT(1-2A)||99.1 ||1.86 ||1.81 |
|} | |} | ||
</div> | </div> | ||
| - | + | ||
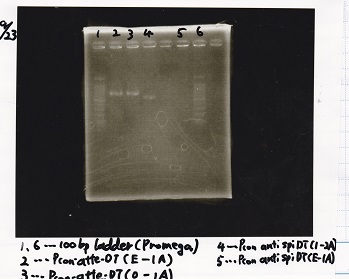
===Electrophoresis=== | ===Electrophoresis=== | ||
| Line 179: | Line 178: | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1||100bp ladder|| || | + | |1||100bp ladder||--||-- |
|- | |- | ||
| - | |2||9/8 Pcon-pT181 | + | |2||9/8 Pcon-pT181 antisense(E-1A)||--||-- |
|- | |- | ||
| - | |3||8/20 Ptet(1)(2-S)|| || | + | |3||8/20 Ptet(1)(2-S)||--||-- |
|- | |- | ||
| - | |4||9/16 Plac2(E-1A)|| || | + | |4||9/16 Plac2(E-1A)||--||-- |
|} | |} | ||
[[File:igku_9231.png]]<br> | [[File:igku_9231.png]]<br> | ||
| Line 191: | Line 190: | ||
===Transformation=== | ===Transformation=== | ||
| - | + | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No Name</span> | <span class="author">No Name</span> | ||
| Line 201: | Line 200: | ||
|} | |} | ||
</div> | </div> | ||
| - | + | ||
===Ligation=== | ===Ligation=== | ||
| - | + | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No Name</span> | <span class="author">No Name</span> | ||
| Line 211: | Line 210: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment||9/19 Plac | + | |experiment||9/19 Plac SpeI+PstI||24.6 µg/mL||9/18 ape12_1R-DT XbaI+PstI||4.5 µg/mL||2 µg/mL |
|- | |- | ||
| - | |experiment||9/21 RBS-GFP-DT | + | |experiment||9/21 RBS-GFP-DT EcoRI+XbaI||88.4 µg/mL||9/15 Pcon-pT181 attenuator EcoRI+SpeI||21 µg/mL||2 µg/mL |
|- | |- | ||
| - | |experiment||9/13 PSB1C3 | + | |experiment||9/13 PSB1C3 XbaI+PstI||26.0 µg/mL||9/16 pT181 attenuator XbaI+PstI||8.2 µg/mL||2 µg/mL |
|} | |} | ||
</div> | </div> | ||
| - | |||
Latest revision as of 06:51, 27 September 2013
Contents |
Sep 23
Transformation
| Name | Sample | Competent Cells | Plate |
|---|---|---|---|
| 9/14 pSB1C3+PkaiBC | 2 µL | 20 µL | -- |
| 9/14 pSB1C3+RpaB | 2 µL | 20 µL | -- |
| 9/14 pkaiBC+Rbs-GFP-DT | 2 µL | 20 µL | -- |
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 3-4 | Pcon-RBS-tetR-PT |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-PT181 anti EcoRI+SpeI | 46.7 | 1.06 | 0.93 |
| Pcon-RBS-tetR-DT EcoRI+XbaI | 119.3 | 0.99 | |
| RBS-8FP-DT EcoRI+XbaI | 87.7 | 1.00 | 0.98 |
| ap42_1R-DT EcoRI+XbaI | 68.8 | 1.32 | 1.03 |
| Pcon-RBS-tetR-DT 1-2 A | 203.0 | 1.66 | 1.79 |
| Pcon-RBS-tetR-DT 3-2 A | 67.7 | 2.64 | 1.28 |
| Pcon-RBS-GFP-DT 1-S A | 185.6 | 1.88 | 2.04 |
| Pcon-RBS-GFP-DT 2-S A | 298.6 | 1.68 | 1.55 |
| Pcon-tetR-DT E-2 A | 202.4 | 1.57 | 1.66 |
PCR
| genome DNA | KOD plus | 10x buffer | dNTP | MgSO4 | SasA_fwd primer | SasA_rev primer | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 9/8 Pcon-pT181 anti | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| 8/20 Ptet(1) | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| 9/16 Plac 2 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94 °C | 98 °C | 57 °C | 68 °C | -- |
| 2min | 10sec | 30sec | 20sec | 30cycles |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/21 Pcon-RBS-GFP-DT EcoRI+XbaI | 47.5 µg/mL | 9/15 Pcon-RBS-tetR-DT EcoRI+SpeI | 59 µg/mL | 2.3 µg/mL |
| experiment | 9/21 Pcon-RBS-GFP-DT EcoRI+XbaI | 47.5 µg/mL | 9/15 Pcon-apt12_1R-DT EcoRI+SpeI | 17 µg/mL | 2.4 µg/mL |
| experiment | 9/21 Pcon-RBS-GFRDT EcoRI+XbaI | 2.1 µg/mL | 9/15 Pcon-DT181 attenuator-DT | 21 µg/mL | 3.0 µg/mL |
| experiment | 9/22 apt 12_1R-DT EcoRI+XbaI | 67.8 µg/mL | 9/18 Pcon-DT181 attenuator EcoRI+SpeI | 5.7 µg/mL | 3.5 µg/mL |
| experiment | 9/22RBS-GFP-DT EcoRI+XbaI | 87.7 µg/mL | 9/18 Pcon-DT18 attenuator EcoRI+SpeI | 6.7 µg/mL | 3.5 µg/mL |
| experiment | 9/13 DT EcoRI+XbaI | 25.4 µg/mL | 9/22 Pcon-DT181 antisense EcoRI+SpeI | 46.8 µg/mL | 2.3 µg/mL |
PCR
| genome DNA | KOD plus | 10x buffer | dNTP | MgSO4 | SasA_fwd primer | SasA_rev primer | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| Pcon-attenuator-DT (E-1A) | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 77.5 | 25 |
| Pcon-attenuator-DT (2-1A) | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 77.5 | 25 |
| Pcon-antisense-spinach-DT (1-2A) | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 77.5 | 25 |
| Pcon-antisense-spinach-DT (E-1A) | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 77.5 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94 °C | 98 °C | 57 °C | 68 °C | -- |
| 2min | 10s | 30s | 45s | 30cycle |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/22 Plac-DT181 attenuator-1 | 204.0 | 2.00 | 1.95 |
| 9/22 Plac-DT181 attenuator-2 | 195.3 | 1.84 | 1.62 |
| 9/22 Plac-DT181 antisense-1 | 188.2 | 1.87 | 2.15 |
| 9/22 Plac-DT181 antisense-2 | 165.0 | 1.95 | 2.66 |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1,6 | 100bp ladder(Promega) | -- | -- |
| 2 | Pcon-attenuator-DT (E-1A) | -- | -- |
| 3 | Pcon-attenuator-DT (2-1A) | -- | -- |
| 4 | Pcon-antisense-spinach-DT (1-2A) | -- | -- |
| 5 | Pcon-antisense-spinach-DT (E-1A) | -- | -- |
Gel Extraction
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pcon-pT181 attenuator-DT(E-1A) | 166.7 | 1.51 | 0.99 |
| Pcon-pT181 attenuator-DT(2-1A) | 122.1 | 1.75 | 1.20 |
| Pcon-pT181 antisense-spinach-DT(1-2A) | 99.1 | 1.86 | 1.81 |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | 9/8 Pcon-pT181 antisense(E-1A) | -- | -- |
| 3 | 8/20 Ptet(1)(2-S) | -- | -- |
| 4 | 9/16 Plac2(E-1A) | -- | -- |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
| pSB1C3 | 2µL | 20µL | 22µL |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/19 Plac SpeI+PstI | 24.6 µg/mL | 9/18 ape12_1R-DT XbaI+PstI | 4.5 µg/mL | 2 µg/mL |
| experiment | 9/21 RBS-GFP-DT EcoRI+XbaI | 88.4 µg/mL | 9/15 Pcon-pT181 attenuator EcoRI+SpeI | 21 µg/mL | 2 µg/mL |
| experiment | 9/13 PSB1C3 XbaI+PstI | 26.0 µg/mL | 9/16 pT181 attenuator XbaI+PstI | 8.2 µg/mL | 2 µg/mL |
Colony PCR
| Sample | base pair |
|---|---|
| 9/22 Pcon-PT181 attenuator12-1R-DT(1~8) | -- |
| 9/22 Pcon-PT181 attenuator(1~4) | -- |
| 9/20 Plac+aptamer12-1R-DT(1~4) | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 54s | 30cycle |
 "
"