Team:Tokyo Tech/Experiment/Crosstalk Confirmation Assay
From 2013.igem.org
ShuntaSuzuki (Talk | contribs) |
|||
| (31 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{tokyotechmenudark}} | {{tokyotechmenudark}} | ||
<div id="text-area"><br> | <div id="text-area"><br> | ||
| - | + | <div class="box" id="title"> | |
| - | < | + | <p style="line-height:0em; text-indent:0em;" name="top">Crosstalk Confirmation Assay</p> |
| - | </ | + | </div> |
| - | < | + | <div class="box"> |
| - | </ | + | <h1>1. Introduction </h1> |
| + | |||
<h2> | <h2> | ||
| - | <p>Our goal in this project is to construct a system to | + | <p>Our goal in this project is to construct a system to circumvent crosstalk by 3OC12HSL-LasR complex on lux promoter. We thought that we could prove that our system precisely works only after we obtain data of crosstalk happening by ourselves. Therefore, we confirmed that crosstalk really happened by the following assay. |
</p> | </p> | ||
</h2> | </h2> | ||
| - | < | + | <h1>2. Summary of the experiment </h1> |
| - | </ | + | |
<h2> | <h2> | ||
| - | <p>Our purpose is to confirm 3OC12HSL-LasR complex really activates lux promoter. | + | <p>Our purpose is to confirm 3OC12HSL-LasR complex really activates lux promoter. We prepared four plasmid sets shown in below (Fig. 3-1-1). We checked what would happen when we added intercellular molecules 3OC6HSL and 3OC12HSL to these plasmid sets. |
</p> | </p> | ||
We prepared twelve conditions as follow.<br> | We prepared twelve conditions as follow.<br> | ||
| - | A-1) Culture containing Ptrc-lasR and Plas-GFP cell with 3OC6HSL induction< | + | <blockquote> |
| - | A-2) Culture containing Ptrc-lasR and Plas-GFP cell with 3OC12HSL induction< | + | <li>A-1) Culture containing Ptrc-<i>lasR</i> and Plas-<i>GFP</i> cell with 3OC6HSL induction |
| - | A-3) Culture containing | + | <li>A-2) Culture containing Ptrc-<i>lasR</i> and Plas-<i>GFP</i> cell with 3OC12HSL induction |
| - | <br> | + | <li>A-3) Culture containing Ptrc-<i>lasR</i> and Plas-<i>GFP</i> cell with DMSO ( no induction) |
| - | B-1) Culture containing Ptrc-lasR and Plux-GFP cell with 3OC6HSL induction< | + | <br><br> |
| - | B-2) Culture containing Ptrc-lasR and Plux-GFP cell with 3OC12HSL induction< | + | <li>B-1) Culture containing Ptrc-<i>lasR</i> and Plux-<i>GFP</i> cell with 3OC6HSL induction |
| - | B-3) Culture containing Ptrc-lasR and Plux-GFP cell with DMSO (no induction)<br> | + | <li>B-2) Culture containing Ptrc-<i>lasR</i> and Plux-<i>GFP</i> cell with 3OC12HSL induction |
| - | <br> | + | <li>B-3) Culture containing Ptrc-<i>lasR</i> and Plux-<i>GFP</i> cell with DMSO (no induction) |
| - | C-1) Culture containing Ptrc-luxR and Plas-GFP cell with 3OC6HSL induction< | + | <br><br> |
| - | C-2) Culture containing Ptrc-luxR and Plas-GFP cell with 3OC12HSL induction< | + | <li>C-1) Culture containing Ptrc-<i>luxR</i> and Plas-<i>GFP</i> cell with 3OC6HSL induction |
| - | C-3) Culture containing Ptrc-luxR and Plas-GFP cell with DMSO (no induction)<br> | + | <li>C-2) Culture containing Ptrc-<i>luxR</i> and Plas-<i>GFP</i> cell with 3OC12HSL induction |
| - | <br> | + | <li>C-3) Culture containing Ptrc-<i>luxR</i> and Plas-<i>GFP</i> cell with DMSO (no induction) |
| - | D-1) Culture containing Ptrc-luxR and Plux-GFP cell with 3OC6HSL induction< | + | <br><br> |
| - | D-2) Culture containing Ptrc-luxR and Plux-GFP cell with 3OC12HSL induction< | + | <li>D-1) Culture containing Ptrc-<i>luxR</i> and Plux-<i>GFP</i> cell with 3OC6HSL induction |
| - | D-3) Culture containing Ptrc-luxR and Plux-GFP cell with DMSO (no induction)<br> | + | <li>D-2) Culture containing Ptrc-<i>luxR</i> and Plux-<i>GFP</i> cell with 3OC12HSL induction |
| - | <br> | + | <li>D-3) Culture containing Ptrc-<i>luxR</i> and Plux-<i>GFP</i> cell with DMSO (no induction) |
| - | Positive control and negative control are similarly operated.< | + | <br><br> |
| + | <li>Positive control and negative control are similarly operated. | ||
| + | </blockquote> | ||
</h2> | </h2> | ||
| - | < | + | |
| - | </ | + | <h1>3. Prediction</h1> |
<h2> | <h2> | ||
| - | <p>If the GFP expression level of B-2) is as high as that of A-2), it | + | <p>If the GFP expression level of B-2) is as high as that of A-2), it shows that 3OC12HSL-LasR complex crosstalk happened. |
</p> | </p> | ||
</h2> | </h2> | ||
| - | < | + | <h1>4. Materials and Methods </h1> |
| - | </ | + | <h3>4-1. Construction </h3> |
| - | < | + | |
| - | </ | + | |
<h2> | <h2> | ||
| - | <p>We used | + | <p>We used <i>lasR</i> or <i>luxR</i> with constitutive promoter as a regulator and <i>GFP</i> understream of <i>las</i> promoter or <i>lux</i> promoter as a reporter. Making pairs from 2 regulators and 2 repressors, we had to prepare four plasmid sets. These sets are transformed into different cells (Gray KM et al., 1994). |
</p> | </p> | ||
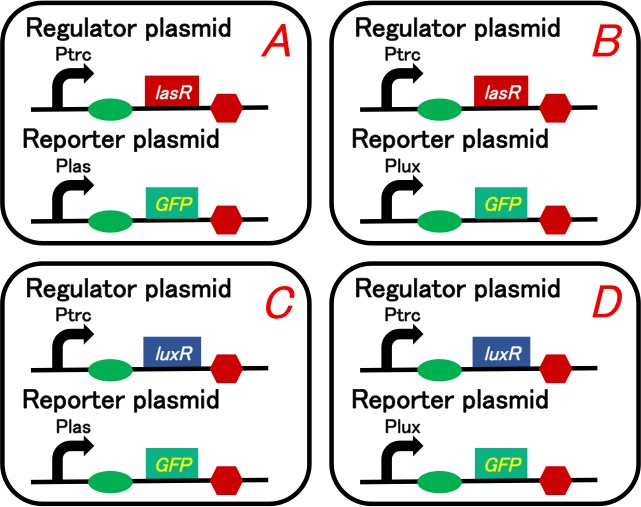
| - | [[Image:Titech2013_CrosstalkConfirmationAssay_3-1_1.jpg|500px|thumb|center|Fig. | + | [[Image:Titech2013_CrosstalkConfirmationAssay_3-1_1.jpg|500px|thumb|center|Fig. 3-1-1. Circuit of the crosstalk confirmation assay]] |
| - | <p>To construct plasmids | + | <p>To construct plasmids above, we ligated Ptrc-RBS-<i>lasR</i>-TT or Ptrc-RBS-<i>luxR</i>-TT as the regulator, and Plas-RBS-<i>GFP</i>-TT or Plux-RBS-<i>GFP</i>-TT as the reporter plasmid.<blockquote> |
| - | + | <li>Regulator: pSB6A1-Ptrc-<i>lasR</i> / Reporter: pSB3K3-Plas-<i>GFP</i> (JM2.300)…Ptrc-<i>lasR</i> and Plas-<i>GFP</i> cell | |
| - | + | <li>Regulator: pSB6A1-Ptrc-<i>lasR</i> / Reporter: pSB3K3-Plux-<i>GFP</i> (JM2.300)…Ptrc-<i>lasR</i> and Plux-<i>GFP</i> cell | |
| - | + | <li>Regulator: pSB6A1-Ptrc-<i>luxR</i> / Reporter: pSB3K3-Plas-<i>GFP</i> (JM2.300)…Ptrc-<i>luxR</i> and Plas-<i>GFP</i> cell | |
| - | + | <li>Regulator: pSB6A1-Ptrc-<i>luxR</i> / Reporter: pSB3K3-Plux-<i>GFP</i> (JM2.300)…Ptrc-<i>luxR</i> and Plux-<i>GFP</i> cell | |
| - | + | <li>pSB6A1-Ptet-<i>GFP</i> (JM2.300)…positive control | |
| - | + | <li>pSB6A1-Promoterless-<i>GFP</i> (JM2.300)…negative control | |
| - | </p></h2> | + | </blockquote></p></h2> |
| - | < | + | <h3>4-2. Strain </h3> |
<h2> | <h2> | ||
| - | | + | JM2.300 |
</h2> | </h2> | ||
| - | < | + | <h3>4-3. Protocol </h3> |
| - | Protocol</ | + | |
<h2> | <h2> | ||
| - | < | + | <blockquote> |
| - | 1. O/N -> FC -> Induction< | + | <b>1. O/N -> FC -> Induction</b> |
| - | + | </blockquote> | |
| - | + | ||
| - | + | <p><blockquote> | |
| - | + | 1.1 Prepare overnight culture of each cell (GFP posi, GFP nega, and samples) at 37°C for 12 h. <br> (=> O/N) | |
| - | + | </blockquote></p> | |
| - | + | <p><blockquote> | |
| - | + | 1.2 Take 30 µL (from GFP posi, GFP nega, sample) of the overnight culture of inducer cell into LB (3 mL) + antibiotics (Amp 50 µg/mL+ Kan 30 µg/mL).<br> (=> Fresh Culture) | |
| - | + | </blockquote></p> | |
| - | </p> | + | <p><blockquote> |
| + | 1.3 Incubate the flesh culture of cells (GFP posi, GFP nega, sample) until the observed OD600 reaches around 0.50. | ||
| + | </blockquote></p> | ||
| + | <p><blockquote> | ||
| + | 1.4 Dilute the flesh culture by the following conditions: | ||
| + | <blockquote>LB (3 mL) + antibiotics (Amp 50 µg/mL + Kan 30 µg/mL)<br> + 5 µM 3OC6HSL (3 µL)</blockquote> | ||
| + | <blockquote>LB (3 mL) + antibiotics (Amp 50 µg/mL + Kan 30 µg/mL)<br> + 5 µM 3OC12HSL (3 µL)</blockquote> | ||
| + | <blockquote>LB (3 mL) + antibiotics (Amp 50 µg/mL + Kan 30 µg/mL)<br> + 5 µM DMSO (3 µL)</blockquote> | ||
| + | </blockquote></p> | ||
| + | |||
| + | <p><blockquote> | ||
| + | 1.5 Incubate the flesh culture of diluted inducer cell for 4 h at 37°C.<br> (=> Induction) | ||
| + | </blockquote></p> | ||
<br> | <br> | ||
| - | < | + | |
| - | 2. Measurement (Flow cytometer)< | + | <blockquote> |
| - | + | <b>2. Measurement (Flow cytometer)</b> | |
| - | + | </blockquote> | |
| - | + | ||
| - | + | <p><blockquote> | |
| - | + | 2.1 Measure all samples' OD600. | |
| - | + | </blockquote></p> | |
| - | + | <p><blockquote> | |
| - | </p> | + | 2.2 Dilute all samples with 1X PBS to keep OD600 in the range from 0.2 to 0.5. |
| + | </blockquote></p> | ||
| + | <p><blockquote> | ||
| + | 2.3 Take 1 mL (from all samples) into a disposable tube (for flow cytometer). | ||
| + | </blockquote></p> | ||
| + | <p><blockquote> | ||
| + | 2.4 Centrifuge them at 9,000g, 4°C, 1 min. and take their supernatant away. | ||
| + | </blockquote></p> | ||
| + | <p><blockquote> | ||
| + | 2.5 Suspend all samples with 1 mL 1X PBS. | ||
| + | </blockquote></p> | ||
| + | <p><blockquote> | ||
| + | 2.6 Measure all samples. | ||
| + | </blockquote></p> | ||
| + | <p><blockquote> | ||
| + | 2.7 Save and organize data. | ||
| + | </blockquote></p> | ||
</h2> | </h2> | ||
| - | < | + | <h1>5. Results of the assay </h1> |
| - | </ | + | |
| - | + | ||
| - | + | ||
<h2> | <h2> | ||
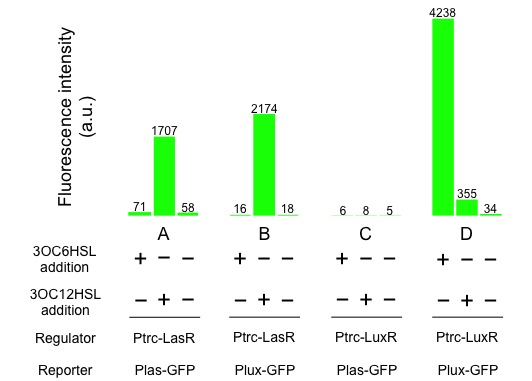
| - | + | [[Image:Titech2013_CrosstalkConfirmationAssay_3-1_2.jpg|500px|thumb|center|Fig. 3-1-2. Crosstalk confirmation results]] | |
| - | + | ||
</h2> | </h2> | ||
| - | |||
| - | |||
<h2> | <h2> | ||
| - | 1. Gray KM, Passador L (1994) Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. Journal of bacteriology 176(10): 3076–3080. | + | <p>Fig. 3-1-2 shows the following, <i>las</i> promoter is activated by 3OC12HSL-LasR complex. Similarly, <i>lux</i> promoter is activated by 3OC12HSL-LasR complex, too. From this result we confirmed that crosstalk by 3OC12HSL-LasR complex really happened. |
| + | </p> | ||
| + | </h2> | ||
| + | <h1>6. Reference </h1> | ||
| + | <h2><OL><LI> | ||
| + | Gray KM, Passador L (1994) Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. Journal of bacteriology 176(10): 3076–3080.</LI></OL> | ||
</h2> | </h2> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div><br> | </div><br> | ||
| + | <html><div align="center"><a href="https://2013.igem.org/Team:Tokyo_Tech/Experiment/Crosstalk_Confirmation_Assay#top"><img src="https://static.igem.org/mediawiki/2013/f/f0/Titeh2013_backtotop.png" width="200px"></a></div></html> | ||
</div> | </div> | ||
Latest revision as of 02:59, 29 October 2013
Crosstalk Confirmation Assay
Contents |
1. Introduction
Our goal in this project is to construct a system to circumvent crosstalk by 3OC12HSL-LasR complex on lux promoter. We thought that we could prove that our system precisely works only after we obtain data of crosstalk happening by ourselves. Therefore, we confirmed that crosstalk really happened by the following assay.
2. Summary of the experiment
Our purpose is to confirm 3OC12HSL-LasR complex really activates lux promoter. We prepared four plasmid sets shown in below (Fig. 3-1-1). We checked what would happen when we added intercellular molecules 3OC6HSL and 3OC12HSL to these plasmid sets.
We prepared twelve conditions as follow.
A-1) Culture containing Ptrc-lasR and Plas-GFP cell with 3OC6HSL induction A-2) Culture containing Ptrc-lasR and Plas-GFP cell with 3OC12HSL induction A-3) Culture containing Ptrc-lasR and Plas-GFP cell with DMSO ( no induction)
B-1) Culture containing Ptrc-lasR and Plux-GFP cell with 3OC6HSL induction B-2) Culture containing Ptrc-lasR and Plux-GFP cell with 3OC12HSL induction B-3) Culture containing Ptrc-lasR and Plux-GFP cell with DMSO (no induction)
C-1) Culture containing Ptrc-luxR and Plas-GFP cell with 3OC6HSL induction C-2) Culture containing Ptrc-luxR and Plas-GFP cell with 3OC12HSL induction C-3) Culture containing Ptrc-luxR and Plas-GFP cell with DMSO (no induction)
D-1) Culture containing Ptrc-luxR and Plux-GFP cell with 3OC6HSL induction D-2) Culture containing Ptrc-luxR and Plux-GFP cell with 3OC12HSL induction D-3) Culture containing Ptrc-luxR and Plux-GFP cell with DMSO (no induction)
Positive control and negative control are similarly operated.
3. Prediction
If the GFP expression level of B-2) is as high as that of A-2), it shows that 3OC12HSL-LasR complex crosstalk happened.
4. Materials and Methods
4-1. Construction
We used lasR or luxR with constitutive promoter as a regulator and GFP understream of las promoter or lux promoter as a reporter. Making pairs from 2 regulators and 2 repressors, we had to prepare four plasmid sets. These sets are transformed into different cells (Gray KM et al., 1994).
To construct plasmids above, we ligated Ptrc-RBS-lasR-TT or Ptrc-RBS-luxR-TT as the regulator, and Plas-RBS-GFP-TT or Plux-RBS-GFP-TT as the reporter plasmid.
Regulator: pSB6A1-Ptrc-lasR / Reporter: pSB3K3-Plas-GFP (JM2.300)…Ptrc-lasR and Plas-GFP cell Regulator: pSB6A1-Ptrc-lasR / Reporter: pSB3K3-Plux-GFP (JM2.300)…Ptrc-lasR and Plux-GFP cell Regulator: pSB6A1-Ptrc-luxR / Reporter: pSB3K3-Plas-GFP (JM2.300)…Ptrc-luxR and Plas-GFP cell Regulator: pSB6A1-Ptrc-luxR / Reporter: pSB3K3-Plux-GFP (JM2.300)…Ptrc-luxR and Plux-GFP cell pSB6A1-Ptet-GFP (JM2.300)…positive control pSB6A1-Promoterless-GFP (JM2.300)…negative control
4-2. Strain
JM2.300
4-3. Protocol
1. O/N -> FC -> Induction
1.1 Prepare overnight culture of each cell (GFP posi, GFP nega, and samples) at 37°C for 12 h.
(=> O/N)
1.2 Take 30 µL (from GFP posi, GFP nega, sample) of the overnight culture of inducer cell into LB (3 mL) + antibiotics (Amp 50 µg/mL+ Kan 30 µg/mL).
(=> Fresh Culture)
1.3 Incubate the flesh culture of cells (GFP posi, GFP nega, sample) until the observed OD600 reaches around 0.50.
1.4 Dilute the flesh culture by the following conditions:LB (3 mL) + antibiotics (Amp 50 µg/mL + Kan 30 µg/mL)
+ 5 µM 3OC6HSL (3 µL)LB (3 mL) + antibiotics (Amp 50 µg/mL + Kan 30 µg/mL)
+ 5 µM 3OC12HSL (3 µL)LB (3 mL) + antibiotics (Amp 50 µg/mL + Kan 30 µg/mL)
+ 5 µM DMSO (3 µL)
1.5 Incubate the flesh culture of diluted inducer cell for 4 h at 37°C.
(=> Induction)
2. Measurement (Flow cytometer)
2.1 Measure all samples' OD600.
2.2 Dilute all samples with 1X PBS to keep OD600 in the range from 0.2 to 0.5.
2.3 Take 1 mL (from all samples) into a disposable tube (for flow cytometer).
2.4 Centrifuge them at 9,000g, 4°C, 1 min. and take their supernatant away.
2.5 Suspend all samples with 1 mL 1X PBS.
2.6 Measure all samples.
2.7 Save and organize data.
5. Results of the assay
Fig. 3-1-2 shows the following, las promoter is activated by 3OC12HSL-LasR complex. Similarly, lux promoter is activated by 3OC12HSL-LasR complex, too. From this result we confirmed that crosstalk by 3OC12HSL-LasR complex really happened.
 "
"