Team:ZJU-China/Project/GhostSensor/Results
From 2013.igem.org
(→Detect thrombin using membrane scaffold FA and FB) |
m (→Subcellular distribution of inner-membrane scaffolds) |
||
| (70 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
</html>{{:Team:ZJU-China/template/project-menu|GhostSensor=Checked}}<html> | </html>{{:Team:ZJU-China/template/project-menu|GhostSensor=Checked}}<html> | ||
<div class="content"> | <div class="content"> | ||
| - | <h2><span class="mw-headline" id="Ghost_Sensor:_Result"> Ghost | + | <h2><span class="mw-headline" id="Ghost_Sensor:_Result"> Bacterial Ghost: Results</span></h2> |
</html> | </html> | ||
__TOC__ | __TOC__ | ||
| - | == Characterize the function of protein E == | + | == '''Characterize the function of protein E''' == |
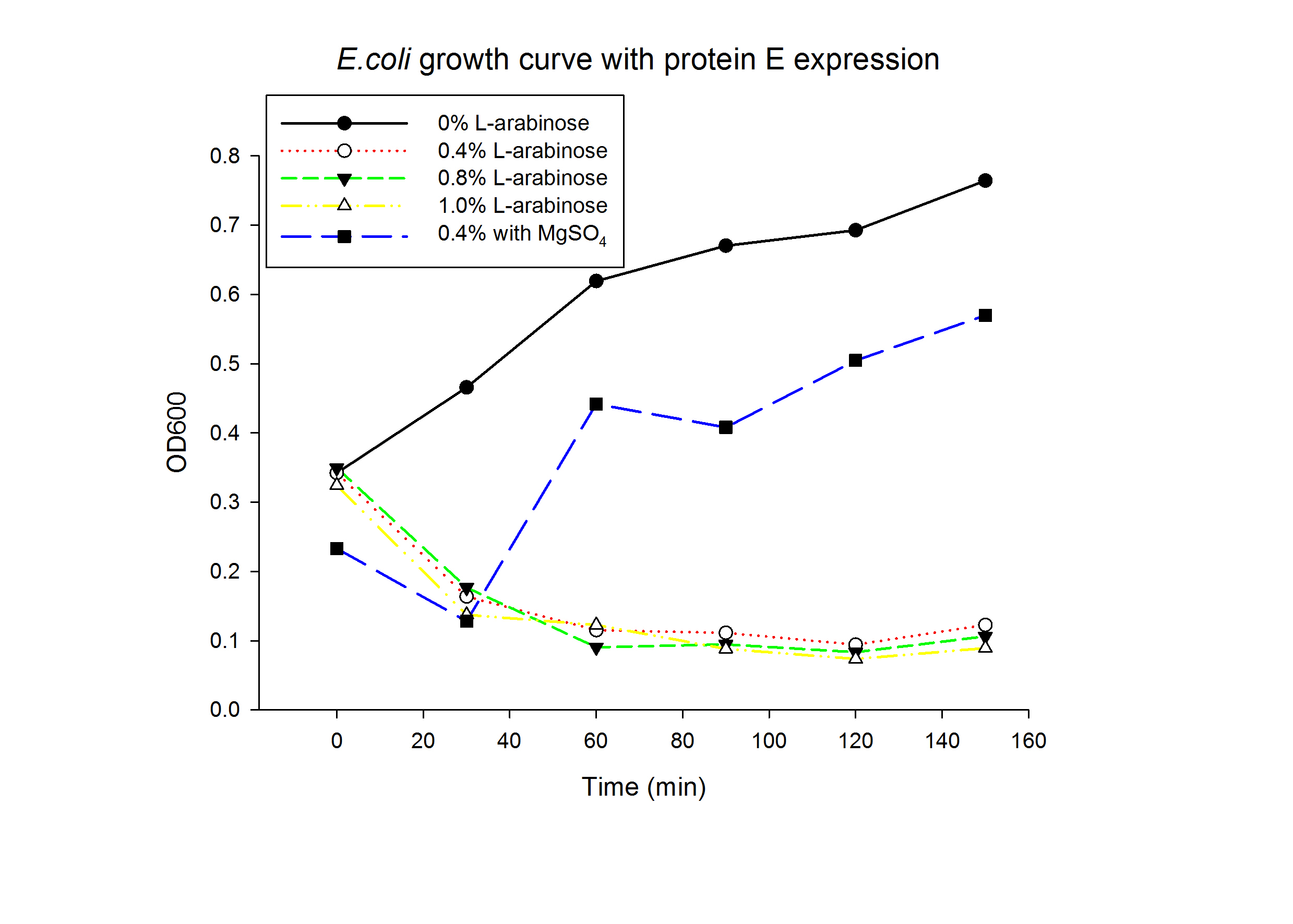
| - | '''1.1''' Protein E was put under the control of pBAD promoter. We recorded the growth curve of transformed E.coli cells treated with different concentrations of L-arabinose varying from 0.2% to 1%. | + | '''1.1''' Protein E was put under the control of pBAD promoter. We recorded the growth curve of transformed E.coli cells treated with different concentrations of L-arabinose varying from 0.2% to 1%. MgSO<sub>4</sub> has been reported to allow the expression of protein E but inhibit lysis. MgSO<sub>4</sub> was added 30min before the induction according to previous works by others. The results showed that even as low as 0.2% L-arabinose can efficiently induce the lysis E.coli. |
| - | <center>[[File: | + | <center>[[File:Pe.JPG|500px]]</center> |
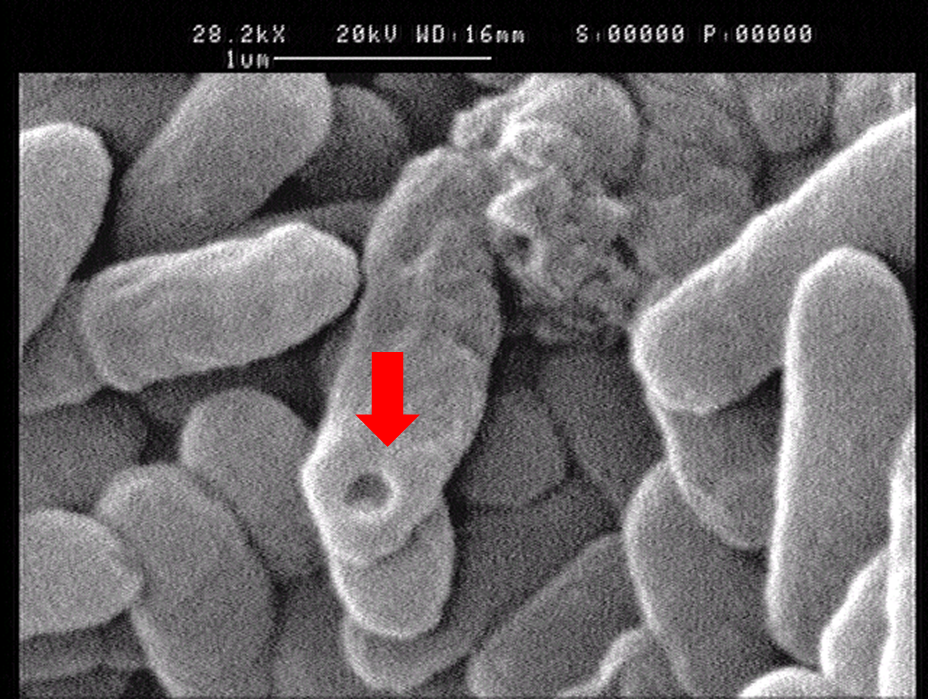
| - | '''1.2''' The lysed products were viewed under SEM and TEM to confirm the formation of tunnels. | + | '''1.2''' The lysed products were viewed under SEM and TEM to confirm the formation of tunnels. MgSO<sub>4</sub> allows production of protein E, but inhibits lysis. 150min later cells were collected by centrifugation and resuspended in water causing immediate lysis by osmotic shock. This procedure would produce holes approximately equal in size to the diameter of the cell. |
[[File:ZJU-ghostsensor-result-2.png|330px|left]] | [[File:ZJU-ghostsensor-result-2.png|330px|left]] | ||
| - | [[File: | + | [[File:Bacterioghostsem2.jpg|330px|right]] |
| - | + | <html><div class="clean"></div></html> | |
| + | <small> | ||
| - | Right: SEM picture of protein E lysed E.coli with | + | <center> |
| + | Left: SEM picture of protein E lysed ''E.coli''. | ||
| + | |||
| + | Right: SEM picture of protein E lysed ''E.coli'' with MgSO<sub>4</sub> protocol. The tunnel is relatively bigger than the left one. | ||
| + | </center> | ||
| + | |||
| + | </small> | ||
<center> | <center> | ||
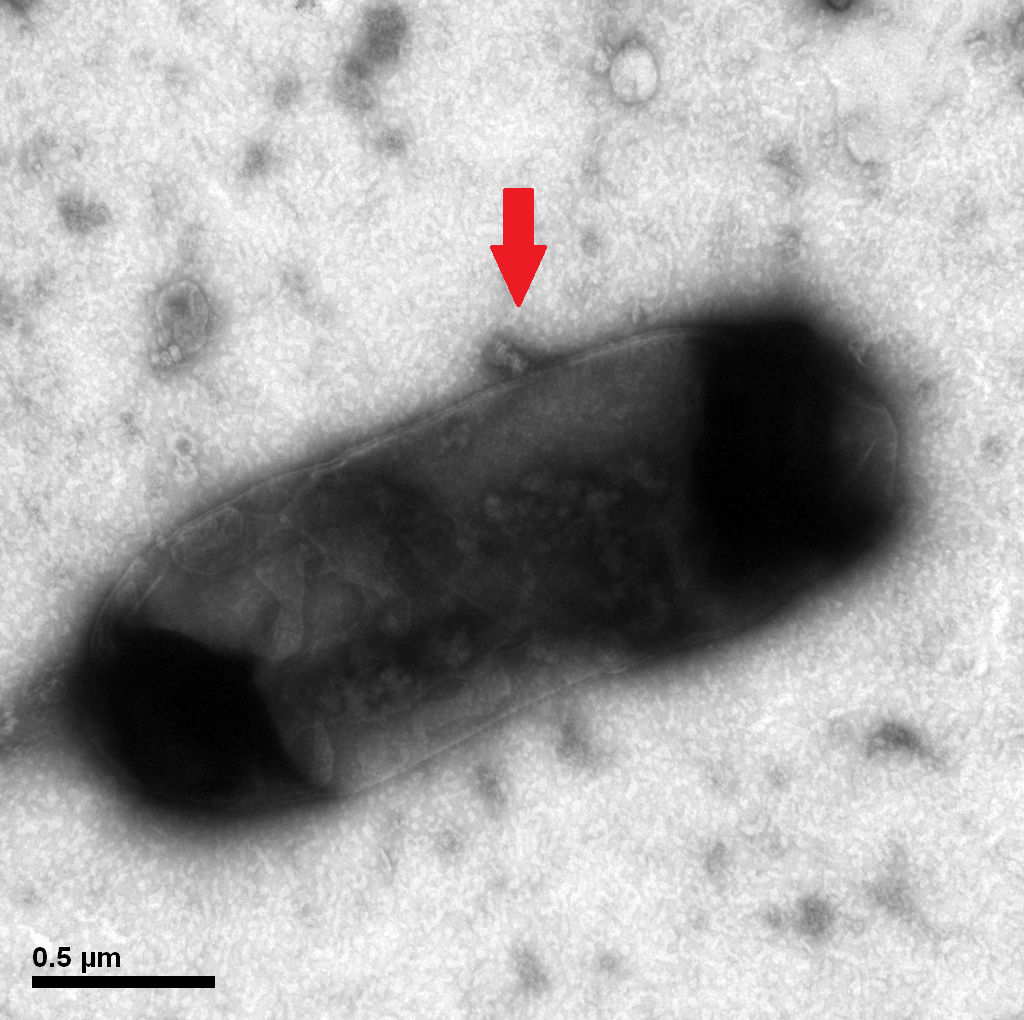
| - | [[File: | + | [[File:3-1-50000X--3.png|300px]] |
| - | + | Protein E mediated lysis of ''E.coli'' under TEM. A presumable efflux of cytoplasmic material is indicated by the arrow. | |
| + | </center> | ||
| + | |||
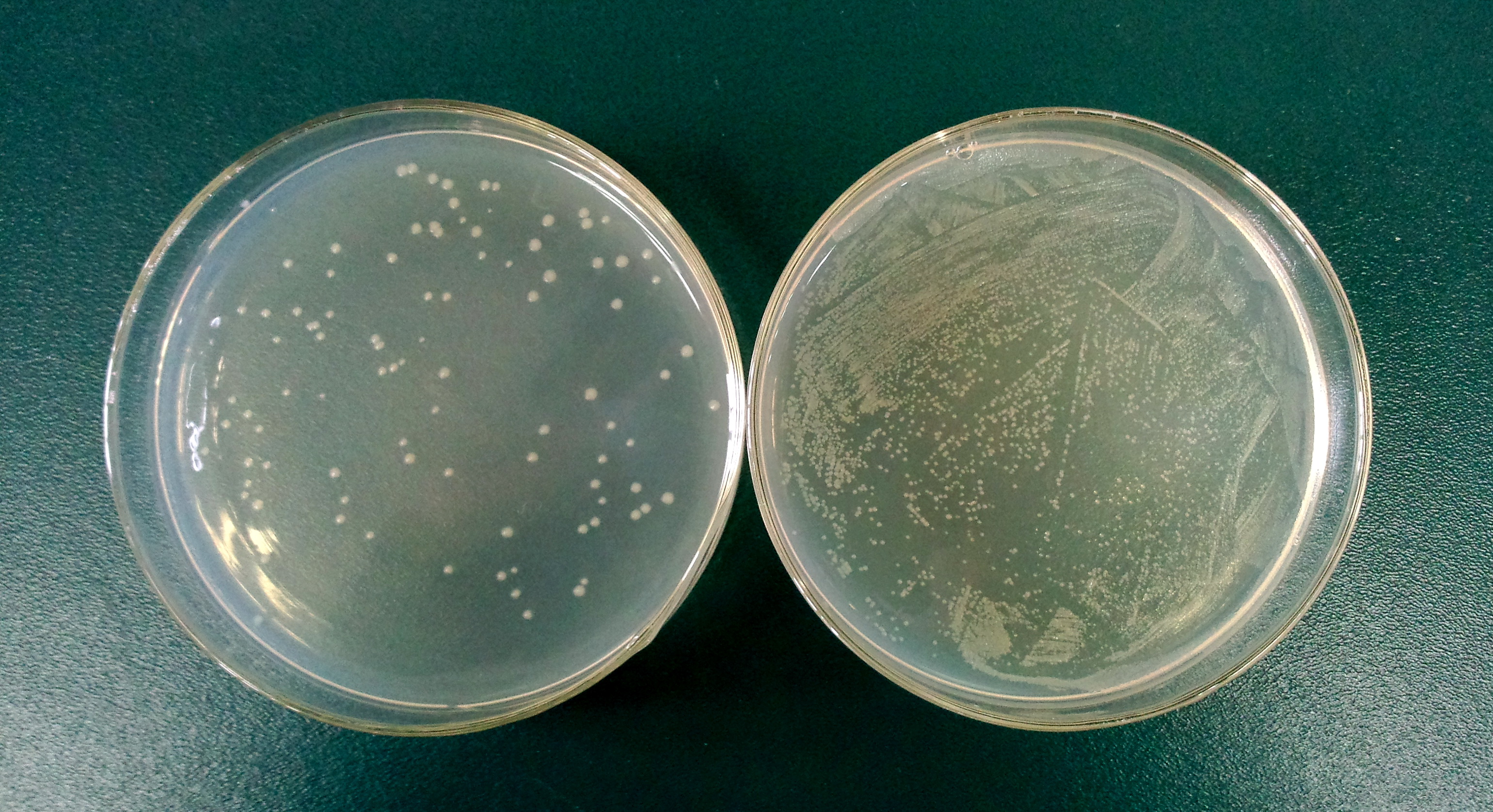
| + | '''1.3''' Determine the lysis ratio of protein E. The bacterial culture was diluted in a ratio 1:10,000 before and after the induction by L-arabinose. Then 100μL was taken to a non-antibotic plate and culture at 37C for 5hrs. The lysis ratio was determined by the equation: lysis ratio=(1-cfu before induction/cfu after induction)*100%. | ||
| + | |||
| + | <center> | ||
| + | [[File:ZJU-Bacterial-Ghost-Results-5.jpg|620px]] | ||
| + | </center> | ||
| + | <center> | ||
| + | <small> | ||
| + | The cells in left plate were induced while the right plate was not. <br> | ||
| + | (The lysis ratio=(1-102/9690)*100%=98.9%) | ||
| + | </small> | ||
</center> | </center> | ||
| - | |||
| - | |||
| - | |||
| + | == '''Characterize the function of aptamers''' == | ||
| + | We chose two well-characterized aptamers, namely 15-mer and 29-mer aptamers, targeting two different sites of thrombin. In order to achieve optimal spatial distance, we linked each of them with either short or long linkers (the underlined sequence below indicates the linker sequence). | ||
| - | + | {| border="1" cellspacing="0" cellpadding="1" align="center" | |
| - | + | !Thrombin aptamers with Short linker: | |
| - | [[File: | + | 5’-biotin-<u>tttttt</u>ggttggtgtggttgg |
| - | [[File:ZJU- | + | |
| + | 5’-biotin-<u>ttt</u>agtccgtggtagggcaggttggggtgact | ||
| + | |||
| + | |} | ||
| + | |||
| + | |||
| + | {| border="1" cellspacing="0" cellpadding="1" align="center" | ||
| + | !Thrombin aptamers with Long linker: | ||
| + | |||
| + | 5’-biotin-<u>tttttttttttt</u>ggttggtgtggttgg | ||
| + | |||
| + | 5’-biotin-<u>ttttttttt</u>agtccgtggtagggcaggttggggtgact | ||
| + | |||
| + | |} | ||
| + | |||
| + | |||
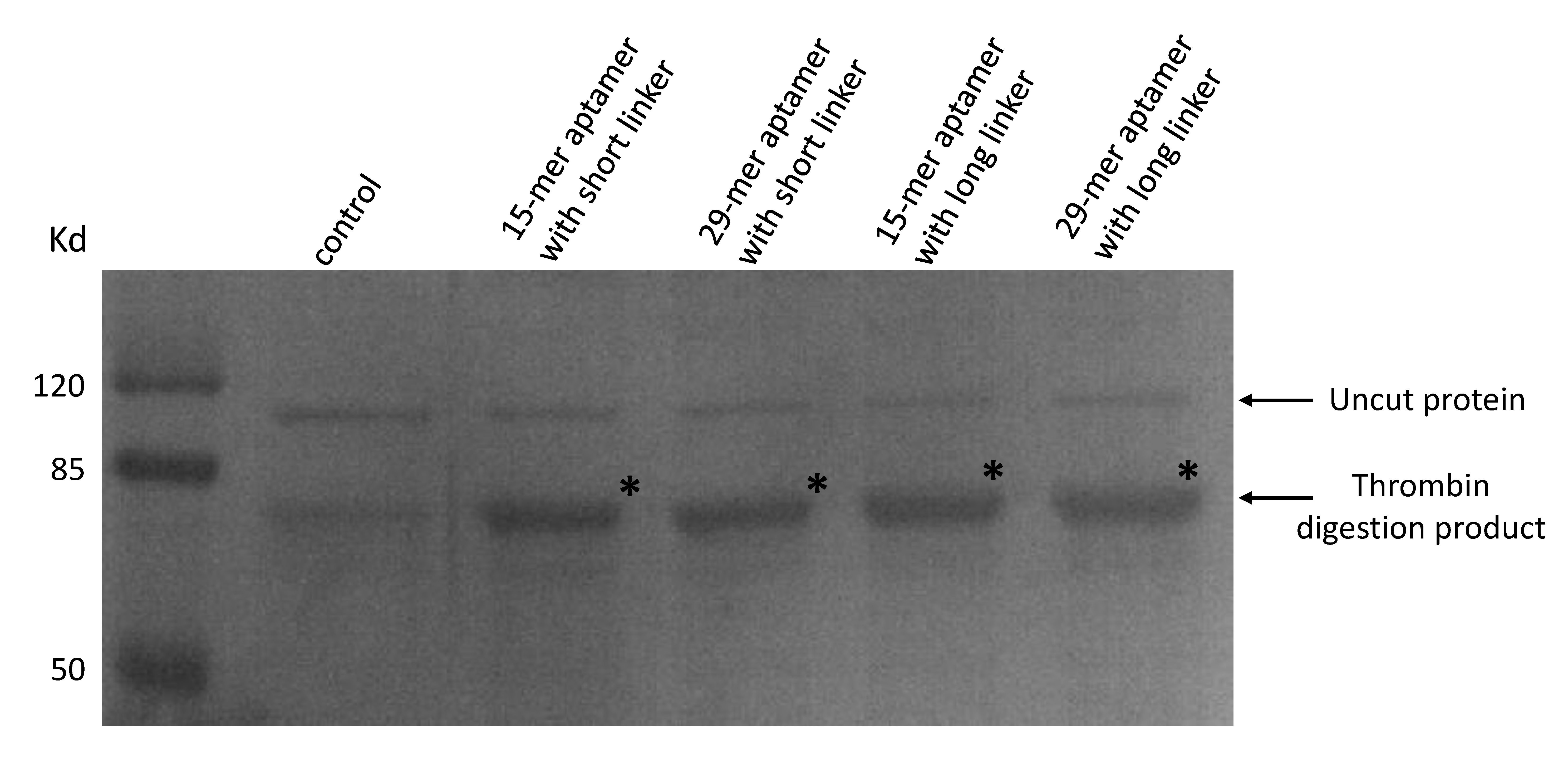
| + | As our aptamers were biotin modified, we used a streptavidin beads pull down assay to see if these aptamers were capable of binding to thrombin. | ||
| + | |||
| + | |||
| + | <center> | ||
| + | [[File:ZJUaptamerpulldown.jpg|600px]] | ||
| + | |||
| + | <small> | ||
| + | (Coomassie blue staining of thrombin digested products. 20μL of streptavidin beads and final concentration of 50nM thrombin were added to a 300μL PBS solution. A final concentration of 50nM 15-mer aptamer with short linker (lane2), 29-mer aptamer with short linker (lane 3), 15-mer aptamer with long linker (lane4) and 29-mer aptamer with long linker (lane 5) were added respectively. No aptamers were added to lane 1. Beads were washed and resuspended and moved to a new solution of purified proteins in which a thrombin cutting site is included to allow digestion.) | ||
| + | </small> | ||
| + | </center> | ||
| + | |||
| + | == '''Subcellular distribution of inner-membrane scaffolds''' == | ||
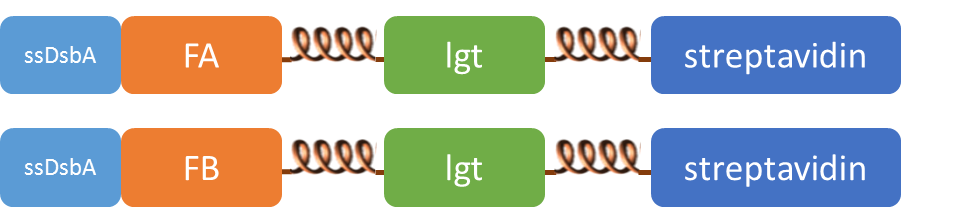
| + | SsDsbA is the signal recognition particle (SRP)-dependent signaling sequence of DsbA. ssDsbA-tagged proteins are thus destined to export to the periplasmic space via the SRP pathway. With ssDsbA fused to the N-terminus, fusion inner-membrane proteins with transmembtrane domain are expected to be anchored onto inner membrane of ''E.coli''. | ||
| + | |||
| + | [[File:ZJU-GhostSensor-4.png|330px]] | ||
| + | [[File:ZJU-GhostSensor-5.png|300px]] | ||
| + | <html><div class="clean"></div></html> | ||
| + | |||
| + | We tried to confirm the localization of our inner-membrane scaffolds by looking at the subcellular distribution of green fluorescence in the cell. However, our confocal microscopy doesn't possess enough resolution to let us distinguish the subcellular localization of the fluorescence. So we resort to another way to tackle this problem. | ||
| + | |||
| + | We believe the nature to be rational and inferable. Logistically, because the construction of bacterial ghost is successful and cytosol would flow out to be replaced by PBS buffer, the fluorescence should come from nowhere but the membrane. In such way, we assume that our protein scaffolds have been successfully anchored to the inner-membrane of ''E. coli''. | ||
| - | |||
<html> | <html> | ||
| + | <div style="float:left;">Previous: | ||
| + | <a href="./Design">Bacterial Ghost: Design</a> | ||
| + | </div> | ||
| + | <div style="float:right;">Next: | ||
| + | <a href="../TheGhostKit/GhostSensor">The Ghost Kit: Ghost Sensor</a> | ||
| + | </div> | ||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
Latest revision as of 03:28, 29 October 2013
Bacterial Ghost: Results
Contents |
Characterize the function of protein E
1.1 Protein E was put under the control of pBAD promoter. We recorded the growth curve of transformed E.coli cells treated with different concentrations of L-arabinose varying from 0.2% to 1%. MgSO4 has been reported to allow the expression of protein E but inhibit lysis. MgSO4 was added 30min before the induction according to previous works by others. The results showed that even as low as 0.2% L-arabinose can efficiently induce the lysis E.coli.
1.2 The lysed products were viewed under SEM and TEM to confirm the formation of tunnels. MgSO4 allows production of protein E, but inhibits lysis. 150min later cells were collected by centrifugation and resuspended in water causing immediate lysis by osmotic shock. This procedure would produce holes approximately equal in size to the diameter of the cell.
Left: SEM picture of protein E lysed E.coli.
Right: SEM picture of protein E lysed E.coli with MgSO4 protocol. The tunnel is relatively bigger than the left one.
Protein E mediated lysis of E.coli under TEM. A presumable efflux of cytoplasmic material is indicated by the arrow.
1.3 Determine the lysis ratio of protein E. The bacterial culture was diluted in a ratio 1:10,000 before and after the induction by L-arabinose. Then 100μL was taken to a non-antibotic plate and culture at 37C for 5hrs. The lysis ratio was determined by the equation: lysis ratio=(1-cfu before induction/cfu after induction)*100%.
The cells in left plate were induced while the right plate was not.
(The lysis ratio=(1-102/9690)*100%=98.9%)
Characterize the function of aptamers
We chose two well-characterized aptamers, namely 15-mer and 29-mer aptamers, targeting two different sites of thrombin. In order to achieve optimal spatial distance, we linked each of them with either short or long linkers (the underlined sequence below indicates the linker sequence).
| Thrombin aptamers with Short linker:
5’-biotin-ttttttggttggtgtggttgg 5’-biotin-tttagtccgtggtagggcaggttggggtgact |
|---|
| Thrombin aptamers with Long linker:
5’-biotin-ttttttttttttggttggtgtggttgg 5’-biotin-tttttttttagtccgtggtagggcaggttggggtgact |
|---|
As our aptamers were biotin modified, we used a streptavidin beads pull down assay to see if these aptamers were capable of binding to thrombin.
(Coomassie blue staining of thrombin digested products. 20μL of streptavidin beads and final concentration of 50nM thrombin were added to a 300μL PBS solution. A final concentration of 50nM 15-mer aptamer with short linker (lane2), 29-mer aptamer with short linker (lane 3), 15-mer aptamer with long linker (lane4) and 29-mer aptamer with long linker (lane 5) were added respectively. No aptamers were added to lane 1. Beads were washed and resuspended and moved to a new solution of purified proteins in which a thrombin cutting site is included to allow digestion.)
Subcellular distribution of inner-membrane scaffolds
SsDsbA is the signal recognition particle (SRP)-dependent signaling sequence of DsbA. ssDsbA-tagged proteins are thus destined to export to the periplasmic space via the SRP pathway. With ssDsbA fused to the N-terminus, fusion inner-membrane proteins with transmembtrane domain are expected to be anchored onto inner membrane of E.coli.
We tried to confirm the localization of our inner-membrane scaffolds by looking at the subcellular distribution of green fluorescence in the cell. However, our confocal microscopy doesn't possess enough resolution to let us distinguish the subcellular localization of the fluorescence. So we resort to another way to tackle this problem.
We believe the nature to be rational and inferable. Logistically, because the construction of bacterial ghost is successful and cytosol would flow out to be replaced by PBS buffer, the fluorescence should come from nowhere but the membrane. In such way, we assume that our protein scaffolds have been successfully anchored to the inner-membrane of E. coli.
 "
"