Team:Buenos Aires/ hybrid
From 2013.igem.org
FedeVignale (Talk | contribs) (→Hybrid promoter induction and repression) |
(→Hybrid promoter induction and repression) |
||
| (14 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
'''Objective''' | '''Objective''' | ||
| - | Characterize the response of the hybrid promoter to the | + | Characterize the response of the hybrid promoter to the AHL/LuxR inducer complex. |
'''Method''' | '''Method''' | ||
| Line 13: | Line 13: | ||
A culture of ''Rhizobium leguminosarum'' was grown overnight (ON), in TY medium, at 30°C. Besides, a culture of E. coli carrying a plasmid that encodes mRFP under a hybrid promoter inducible by the Lux system of ''Vibrio fischeri'' , and repressible by the phage repressor P22 C2 (Bba_K1106000) was also grown ON, at 37°C, in LB with the appropriate antibiotic. As a positive control, a ''Chromobacterium violaceum'' culture was grown at 30°C in LB medium. | A culture of ''Rhizobium leguminosarum'' was grown overnight (ON), in TY medium, at 30°C. Besides, a culture of E. coli carrying a plasmid that encodes mRFP under a hybrid promoter inducible by the Lux system of ''Vibrio fischeri'' , and repressible by the phage repressor P22 C2 (Bba_K1106000) was also grown ON, at 37°C, in LB with the appropriate antibiotic. As a positive control, a ''Chromobacterium violaceum'' culture was grown at 30°C in LB medium. | ||
| - | Afterwards, the three cultures were centrifuged. ''E. coli'' (DH5α strain) and ''Chromobacterium violaceum'' culture’s supernatants were discarded and the pellets were resuspended in ''Rhizobium leguminosarum'' culture’s supernatant, containing | + | Afterwards, the three cultures were centrifuged. ''E. coli'' (DH5α strain) and ''Chromobacterium violaceum'' culture’s supernatants were discarded and the pellets were resuspended in ''Rhizobium leguminosarum'' culture’s supernatant, containing AHL, which would induce mRFP expression in E. coli, and violacein expression in ''Chromobacterium violaceum''. As a negative control, an "E. coli" culture containing the hybrid promoter was kept in the same medium at which it was previously grown. |
| + | |||
| + | Cultures were then grown for 70 hours in this conditions, taking aliquots after 0, 7, 24 and 70 hours of induction. Finally, bacteria were sonicated and fluorescence measured, normalized by cell growth (OD 600nm). | ||
| - | |||
| - | |||
'''Results''' | '''Results''' | ||
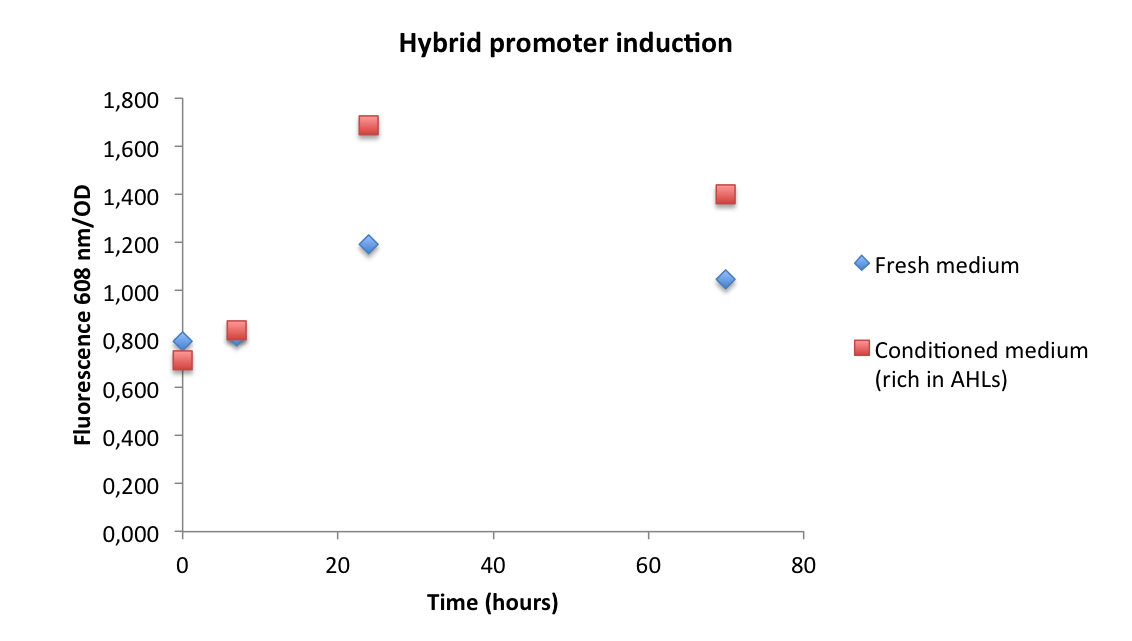
| - | A | + | A greater RFP fluorescence production can be seen in the culture induced with AHL, compared with the negative control. Besides, the culture of ''Chromobacterium violaceum'' turned violet 6 hours after ''Rhizobium leguminosarum'' conditioned medium was added. |
| - | [[File: | + | [[File:IGEMBsAs2013PhibridoNico.png|center|500px]] |
'''Conclusions''' | '''Conclusions''' | ||
| - | According to the results, the hybrid promoter (Bba_K145150) can be induced by | + | According to the results, the hybrid promoter (Bba_K145150) can be induced by AHL-LuxR. However, fresh medium mRFP curve suggests a high basal transcription from this promoter. |
| + | == '''Future experiment: Hybrid promoter induction by AHLs from ''E. coli'' expressing pArs_LuxI''' == | ||
| - | + | '''Objective''' | |
| + | Characterize the induction of the hybrid promoter by AHLs produced by ''E. coli'' carrying our para_LuxI construct. | ||
| + | |||
| + | '''Method''' | ||
| + | Cultures of ''E. coli'' harboring pArs_LuxI will be grown at different concentrations of arsenite. ''E. coli'' containing the plasmid that encodes mRFP under a hybrid promoter (described previously) will also be grown overnight. Next day, bacteria carrying the hybrid promoter will be resuspended in the media conditioned by the ''E. coli'' carrying pArs_LuxI at different concentrations of arsenite. This way, we expect each media to contain different concentrations of AHL according to the arsenite concentration at which bacteria were exposed. | ||
| + | |||
| + | Finally, we will take aliquots at different times to measure RFP induction in each condition. | ||
| + | |||
| + | |||
| + | == '''Future experiment: Hybrid promoter repression''' == | ||
'''Objective''' | '''Objective''' | ||
| Line 38: | Line 48: | ||
'''Method''' | '''Method''' | ||
| - | A culture of Rhizobium leguminosarum will be grown overnight (ON), in TY medium, at 30°C. Besides, a culture of E. coli co-transformed with BBa_K1106000 and the P22 C2 phage repressor under control of an arsenite inducible promoter (Bba_K1106012) will also be grown ON, at 37°C, in LB with the appropriate antibiotic. As a positive control, a Chromobacterium violaceum culture will be grown at 30°C in LB medium. | + | A culture of ''Rhizobium leguminosarum'' will be grown overnight (ON), in TY medium, at 30°C. Besides, a culture of E. coli co-transformed with BBa_K1106000 and the P22 C2 phage repressor under control of an arsenite inducible promoter (Bba_K1106012) will also be grown ON, at 37°C, in LB with the appropriate antibiotic. As a positive control, a ''Chromobacterium violaceum'' culture will be grown at 30°C in LB medium. |
| - | Afterwards, the three cultures will be centrifuged. E. coli and Chromobacterium violaceum culture’s supernatants will be discarded and the pellets resuspended in Rhizobium leguminosarum culture’s supernatant, containing HSL, which would induce mRFP expression in E. coli, and violacein expression in Chromobacterium violaceum. Moreover, E. coli culture will be split in two tubes with different arsenite concentrations (0 ppb and 1000 ppb). | + | Afterwards, the three cultures will be centrifuged. ''E. coli'' and ''Chromobacterium violaceum'' culture’s supernatants will be discarded and the pellets resuspended in Rhizobium leguminosarum culture’s supernatant, containing HSL, which would induce mRFP expression in ''E. coli'', and violacein expression in ''Chromobacterium violaceum''. Moreover, ''E. coli'' culture will be split in two tubes with different arsenite concentrations (0 ppb and 1000 ppb). |
Cultures will then be grown for 72 hours, and aliquots will be taken at 0, 24 and 72 hours of induction. Finally, bacteria will be sonicated and fluorescence measured, normalized by cell growth (OD 600nm). | Cultures will then be grown for 72 hours, and aliquots will be taken at 0, 24 and 72 hours of induction. Finally, bacteria will be sonicated and fluorescence measured, normalized by cell growth (OD 600nm). | ||
| - | |||
| - | |||
</div> | </div> | ||
Latest revision as of 01:30, 28 October 2013
Contents |
Hybrid promoter induction and repression
Hybrid promoter induction
Objective
Characterize the response of the hybrid promoter to the AHL/LuxR inducer complex.
Method
A culture of Rhizobium leguminosarum was grown overnight (ON), in TY medium, at 30°C. Besides, a culture of E. coli carrying a plasmid that encodes mRFP under a hybrid promoter inducible by the Lux system of Vibrio fischeri , and repressible by the phage repressor P22 C2 (Bba_K1106000) was also grown ON, at 37°C, in LB with the appropriate antibiotic. As a positive control, a Chromobacterium violaceum culture was grown at 30°C in LB medium.
Afterwards, the three cultures were centrifuged. E. coli (DH5α strain) and Chromobacterium violaceum culture’s supernatants were discarded and the pellets were resuspended in Rhizobium leguminosarum culture’s supernatant, containing AHL, which would induce mRFP expression in E. coli, and violacein expression in Chromobacterium violaceum. As a negative control, an "E. coli" culture containing the hybrid promoter was kept in the same medium at which it was previously grown.
Cultures were then grown for 70 hours in this conditions, taking aliquots after 0, 7, 24 and 70 hours of induction. Finally, bacteria were sonicated and fluorescence measured, normalized by cell growth (OD 600nm).
Results
A greater RFP fluorescence production can be seen in the culture induced with AHL, compared with the negative control. Besides, the culture of Chromobacterium violaceum turned violet 6 hours after Rhizobium leguminosarum conditioned medium was added.
Conclusions
According to the results, the hybrid promoter (Bba_K145150) can be induced by AHL-LuxR. However, fresh medium mRFP curve suggests a high basal transcription from this promoter.
Future experiment: Hybrid promoter induction by AHLs from E. coli expressing pArs_LuxI
Objective
Characterize the induction of the hybrid promoter by AHLs produced by E. coli carrying our para_LuxI construct.
Method Cultures of E. coli harboring pArs_LuxI will be grown at different concentrations of arsenite. E. coli containing the plasmid that encodes mRFP under a hybrid promoter (described previously) will also be grown overnight. Next day, bacteria carrying the hybrid promoter will be resuspended in the media conditioned by the E. coli carrying pArs_LuxI at different concentrations of arsenite. This way, we expect each media to contain different concentrations of AHL according to the arsenite concentration at which bacteria were exposed.
Finally, we will take aliquots at different times to measure RFP induction in each condition.
Future experiment: Hybrid promoter repression
Objective
Characterize the repression of the hybrid promoter by c2 repressor.
Method
A culture of Rhizobium leguminosarum will be grown overnight (ON), in TY medium, at 30°C. Besides, a culture of E. coli co-transformed with BBa_K1106000 and the P22 C2 phage repressor under control of an arsenite inducible promoter (Bba_K1106012) will also be grown ON, at 37°C, in LB with the appropriate antibiotic. As a positive control, a Chromobacterium violaceum culture will be grown at 30°C in LB medium.
Afterwards, the three cultures will be centrifuged. E. coli and Chromobacterium violaceum culture’s supernatants will be discarded and the pellets resuspended in Rhizobium leguminosarum culture’s supernatant, containing HSL, which would induce mRFP expression in E. coli, and violacein expression in Chromobacterium violaceum. Moreover, E. coli culture will be split in two tubes with different arsenite concentrations (0 ppb and 1000 ppb). Cultures will then be grown for 72 hours, and aliquots will be taken at 0, 24 and 72 hours of induction. Finally, bacteria will be sonicated and fluorescence measured, normalized by cell growth (OD 600nm).
 "
"