Team:Heidelberg/Templates/Del week13 EG
From 2013.igem.org
(Difference between revisions)
(→24-07-2013) |
|||
| (2 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | |||
| + | |||
==24-07-2013== | ==24-07-2013== | ||
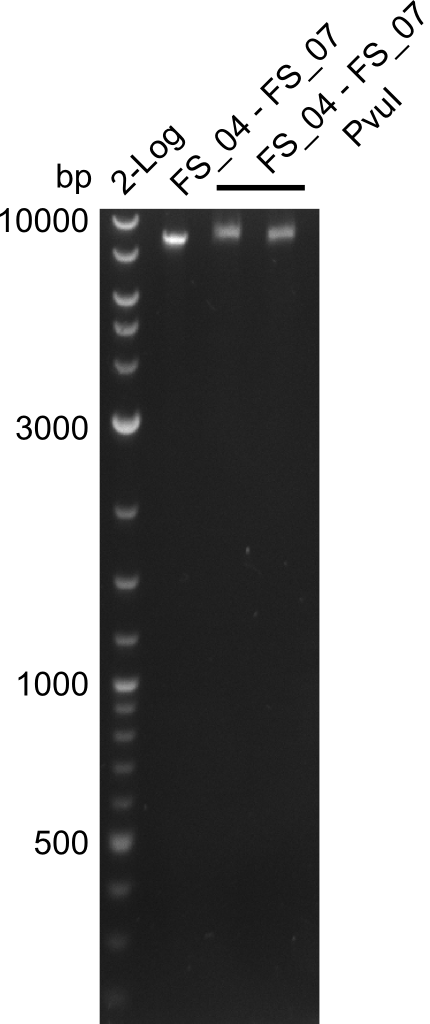
===Restriction digest of fragment FS_04 to FS_07; 11.1 kb with PvuI-HF=== | ===Restriction digest of fragment FS_04 to FS_07; 11.1 kb with PvuI-HF=== | ||
| Line 9: | Line 11: | ||
! what !! µl | ! what !! µl | ||
|- | |- | ||
| - | | FS_04 to FS_07 ( | + | | FS_04 to FS_07 (14-07-2013 and 15-07-2013) || 15 |
|- | |- | ||
| PvuI-HF || 0.8 | | PvuI-HF || 0.8 | ||
Latest revision as of 18:23, 3 October 2013
Contents |
24-07-2013
Restriction digest of fragment FS_04 to FS_07; 11.1 kb with PvuI-HF
Incubation at 37°C for 45 min
| what | µl |
|---|---|
| FS_04 to FS_07 (14-07-2013 and 15-07-2013) | 15 |
| PvuI-HF | 0.8 |
| Buffer CutSmart | 2 |
| dd H2O | 2.8 |
| Expected fragment lengths [bp] | 6187, 4917 |
Results:
- restriction digest did not work
- digest will be repeated with newly amplified and purified DelEG
28-07-2013
Amplification from FS_04 to FS_09 ; 14.4 kb
2 reactions with conditions I and II
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_04: (1/10) | 2 |
| FS_09: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions I
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:40 | |
| 1 | 72 | 13 min |
| 1 | 10 | inf |
- Conditions II
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 4:40 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 4:40 | |
| 1 | 72 | 13 min |
| 1 | 10 | inf |
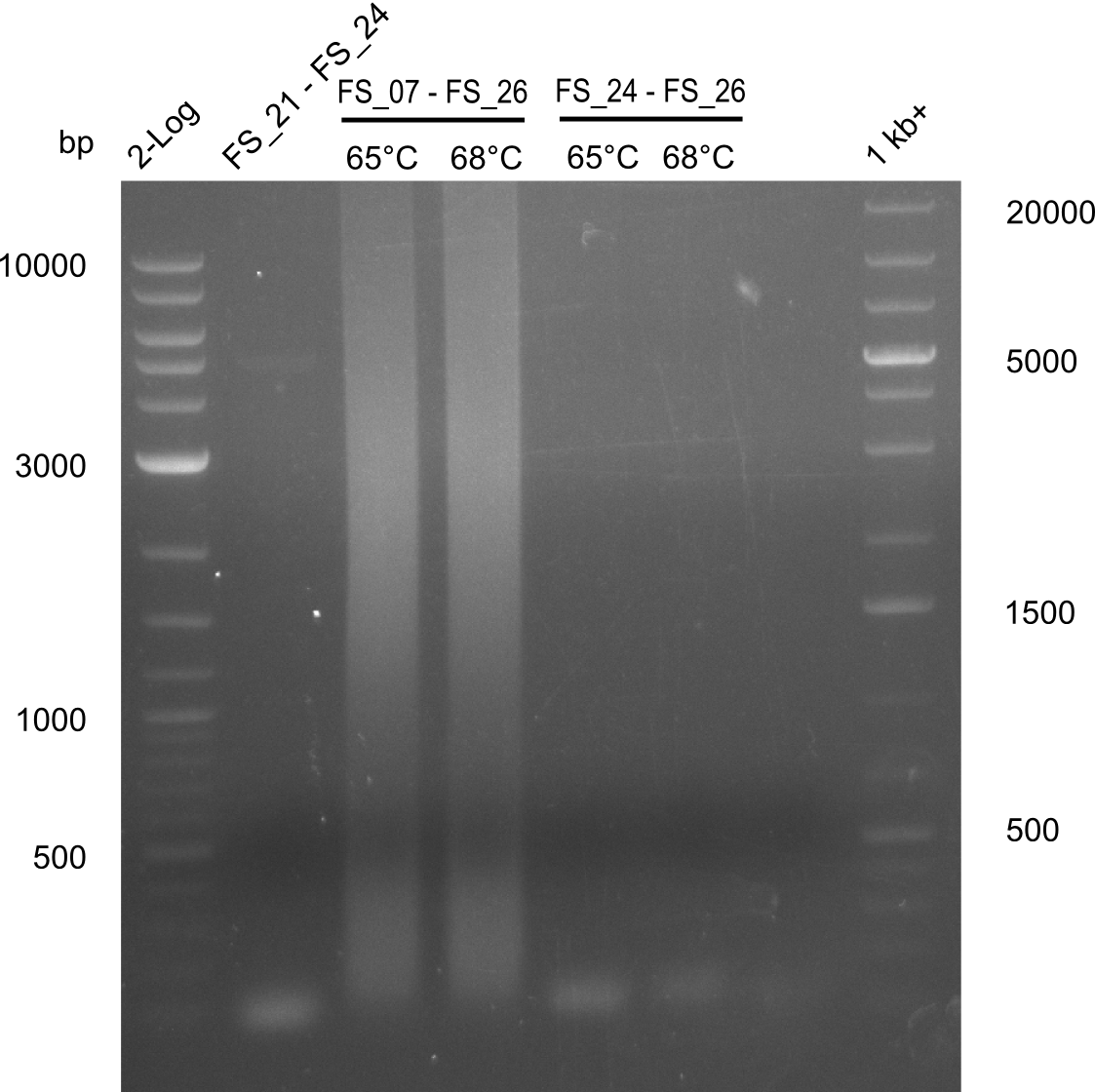
Amplification from FS_26 to FS_07
This amplification did not make sense, two reverse Primer were used. We mixed up Primer FS_24 with Primer FS_26.
2 reactions with conditions I and II
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_26: (1/10) | 2 |
| FS_07: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions I
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 13 min |
| 1 | 10 | inf |
- Conditions II
| Biorad C1000 Touch Block A | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3:20 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 12 min |
| 1 | 10 | inf |
Results:
- Amplification of DelEG did not work, neither with annealing at a constant temperature of 65°C nor with a touchdown starting from 68°C
- later on it was discovered, that primers had been mixed up
Amplification from FS_26 to FS_24
This amplification did not make sense, two reverse Primer were used.
2 reactions with conditions I and II
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_26: (1/10) | 2 |
| FS_24: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions I
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 13 min |
| 1 | 10 | inf |
- Conditions II
| Biorad C1000 Block A | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3:20 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 12 min |
| 1 | 10 | inf |
Results:
- Amplification of DelEG did not work, neither with annealing at a constant temperature of 65°C nor with a touchdown starting from 68°C
- later on it was discovered, that primers had been mixed up
 "
"