Team:Exeter/Modelling
From 2013.igem.org
(→Results) |
|||
| (161 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
<div class="row"> | <div class="row"> | ||
<div class="span" style="text-align:justify"> | <div class="span" style="text-align:justify"> | ||
| + | |||
| + | == Modelling == | ||
__NOTOC__ | __NOTOC__ | ||
| - | == | + | === Abstract === |
| - | |||
| - | + | We have developed a stochastic model of a colour bio-camera system in <i>E.coli</i>. The model takes the light incident of the bacteria and stochastically estimates the light regulated expression of pigment proteins over time. The population of proteins over time is combined with their absorption spectra and plotted as a surface showing the cell’s absorption spectrum over time. There have been insufficient experimental results to test the model with rates and absorption spectra taken from literature and theoretical conjecture. This prevents us from drawing accurate conclusions. However our preliminary results suggest that a collection of cells will show a reliable colour but with a very high variance between individuals. This reduces the spatial control of expression and thus the resolution of any bio-camera. One way to counter this problem and the problem of one pathway dominating the others is to insert multiple copies of the light regulated genes. This causes spatial control to be increased as the behaviour of each individual is more predictable. This model requires experimental data to test it and update it to produce an accurate description of our biological system. In its current for it provides a well documented framework for future rule-based modelling of similar systems. | |
| - | == | + | === Introduction === |
| - | + | Our model predicts how the absorption spectrum of a modified ''E. coli'' changes over time in response to a given incident light spectrum. It can estimate key properties of the system such as stability, development time of the image, and the balance of colours. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | The model is split into three sections. Initially, the reaction of each sensor species to a given incident light spectrum is calculated. This information feeds to the heart of the model, which involves simulating the biochemistry of our designed pathways. Finally the data from the simulation is used to plot the absorption spectrum of the system as it varies with time. | |
| - | + | Currently the model is based on results from literature and theoretical conjecture as sufficient experimental results are not available. The motivation for the model is to numerically characterize our bio-bricks for future use and to help us create the first colour coliroid. With experimental results the model could readily be updated to fulfill its original mandate. | |
| - | + | Control of bacteria using light has many applications. Di-chromatic control of bacteria has been achieved, the challenge now is for Tri-chromatic control. Our lab project aims to produce a full colour ‘coliroid’ to demonstrate tri-chromatic control of bacteria. The modified <i>E.coli</i> are designed to carry three independent light sensitive pathways that each control the production of a specific pigment protein. | |
| - | + | A reliable computer model is essential for accurate implementation of tri-chromatic control. In the case of the theoretical bio-camera it is required to achieve an even balance of colours and optimum development time. In other applications it is essential to have accurate temporal control over the expression of light regulated genes. | |
| - | + | The model is based around KaSim, a stochastic simulator that reads Kappa, a rule based language. Stochastic simulation has the advantage over deterministic simulation because it replicates the random nature of brownian motion in cells more accurately. Rule based languages are particularly useful for biochemical signalling as the physical reactions are readily converted into rules. Matlab is used to process the signal from the incident light to the KaSim simulation. The outputs of the simulation is used in MATLAB to calculate the change in absorbtion spectrum over time. | |
| - | === | + | <!-- === The Team === |
| - | + | <center>'''We are Exeter iGEM's dry team. Together we are responsible for the creation and development of a working model of our system and other various tasks.'''</center> | |
| - | + | <center><TABLE cellpadding="20"> | |
| + | <TR> | ||
| + | <TD> [[File:Exeter-angus-laurenson.jpg|right||250px]] <center>[[User:Angus|Angus Laurenson]]</center> | ||
| + | <TD> [[File:Exeter-callum-vincent.jpg|right||250px]] <center>[[User:Cv235|Callum Vincent]]</center> | ||
| + | <TD> [[File:Exeter-henri-laakso.jpg|right||250px]] <center>[[User:Hlaakso|Henri Laakso]]</center> | ||
| + | </TR> | ||
| + | </TABLE></center> | ||
| + | <center>'''We look forward to seeing you at the European Jamboree.'''</center> --> | ||
| - | == | + | === Modelling Software === |
| - | The | + | The modelling was primarily conducted using two platforms; [http://www.mathworks.co.uk/products/matlab/ MATLAB®] and KaSiM. Together they provide the necessary tools to create an accurate model of our system. |
| - | + | They have been used succesfully by previous iGEM teams, notably the [https://2010.igem.org/Team:Edinburgh/Results Edinburgh 2010] and [https://2010.igem.org/Team:Edinburgh/Results 2011] teams, both of which one Best Model at the European Jamboree. This was the primary reason we chose the Kappa language to write our model in. | |
| - | + | KaSiM is a [http://en.wikipedia.org/wiki/Stochastic stochastic] simulator that executes files written in [http://www.kappalanguage.org/ Kappa]. Kappa is a rule based modelling language for protein interaction networks. To download KaSiM or the manual please visit the [https://github.com/jkrivine/KaSim KaSiM page.] | |
| - | + | ==== KaSiM ==== | |
| - | + | [https://github.com/jkrivine/KaSim KaSiM]is a [http://en.wikipedia.org/wiki/Stochastic stochastic]simulator of rule based models written in the Kappa language. It is open source program that uses a variation of the Gillespie's algorithm to generate statistically correct trajectories. We advise you take the time to read the introduction to KaSiM in the [https://github.com/jkrivine/KaSim/tree/master/man KaSiM manual] . | |
| - | This has two important implications. Firstly it means that the sensor will not be activated by light that has no overlap between its spectrum and the frequency response of the sensor. Secondly there will be a saturation point at high intensity light where increasing the intensity will not appreciably affect the system as the activation rate will dominate the deactivation rate | + | ==== Kappa ==== |
| + | |||
| + | Kappa is a high level rule-based language designed to describe protein interaction networks. Rule-based languages describe a system by a governing set of rules. There is no change in the configuration of the system without the execution of a rule. There are five fundamental parts to the kappa language; agents, tokens, rules, rates and observables. A quick introduction to these concepts is given below. For more detailed information we suggest visiting the [http://www.kappalanguage.org/ Kappa] website or for full examples [http://rulebase.org/ RuleBase] which offers a repository of complete rule based models. We can also recommend the 2012 Edinburgh iGEM team's [https://2012.igem.org/Team:Edinburgh/Modelling/Kappa/Introduction Introduction to Kappa] which proved useful to us early on. | ||
| + | |||
| + | '''Agents and tokens''' | ||
| + | |||
| + | Agents and tokens represent the constituent molecules of a system. Agents are quantified in terms of their population number and are characterised having binding sites. Whereas tokens are quantified in terms of their concentration and do not have binding sites. | ||
| + | |||
| + | '''Rules''' | ||
| + | |||
| + | Rules represent specific bio-chemical processes, and involve either tokens, agents or both. Each rule has an initial condition which must be satisfied for the rule to be valid. If the conditions are met, the rule is applied according to the rate. | ||
| + | |||
| + | '''Rates''' | ||
| + | |||
| + | Each rule has a specific rate constant which determines the number of times the rule is applied for each instance of the rule. | ||
| + | |||
| + | '''Observables''' | ||
| + | |||
| + | Observables determine the output data in terms of either agent count or token concentration. | ||
| + | |||
| + | ==== MATLAB® ==== | ||
| + | |||
| + | [http://www.mathworks.co.uk/products/matlab/ MATLAB®] is a high-level language and interactive environment for numerical computation, visualization, and programming. It can be used to analyse data, develop algorithms, and create models and applications. For guidance on using MATLAB® please see the [http://www.mathworks.co.uk/help/matlab/ mathworks documentation] . | ||
| + | |||
| + | === The model === | ||
| + | |||
| + | The model describes the absorption spectrum of the cell as a function of time and a given time invariant incident light spectrum. This is achieved by calculating the effect of the known light input on the individual light sensitive proteins. This information is then fed into a stochastic simulation of the biological pathways that computes how the population of sensors and pigments vary over time. The total absorption spectrum over time is given by the sum of the product of every protein's population and the corresponding absorption spectrum of the protein. In this section each of these steps in considered in the following subsections. | ||
| + | |||
| + | It will be helpful to read the theory page and the modelling software section of this page before reading further as an understanding of the biological pathways and software is important. | ||
| + | |||
| + | ==== Sensor activation rate calculation ==== | ||
| + | |||
| + | In the rule based model each sensor is thought of as a two-state binary system (on/off). In the on state it catalyses the phosphorylation of the primary intermediate, sending a signal to the downstream pathway. In the off state it does not. | ||
| + | |||
| + | Activation by light either turns a sensor on or off depending on the sensor species; red and blue light sensors are switched off when activated, while the green light sensor is switched on when activated. The activation rate is the forward rate of the rule that governs the state of a sensor. Its counterpart, the deactivation rate is the backward rate of the same rule and is constant. The activation rate is calculated as the integral over all space of the product of the sensor's frequency response with the frequency spectrum of the incident light. | ||
| + | |||
| + | [[Image:ActivationExample1.jpg|thumb|center|upright=5| This images shows the red light sensor (Cph8) being activated by incident light with a wavelength spectrum in the form of a sine wave. The red line shows the absorbtion spectrum of Cph8, the black line is the incident light spectrum and the filled in blue area is the area under the curve of the product between the two. This gives us the activation rate for the sensor which in this case is a very high 913.3643. Such a high rate will cause the sensors to be activated permanently]] | ||
| + | |||
| + | This has two important implications. Firstly it means that the sensor will not be activated by light that has no overlap between its spectrum and the frequency response of the sensor. Secondly there will be a saturation point at high intensity light where increasing the intensity will not appreciably affect the system as the activation rate will dominate the deactivation rate. These characteristics are representative of those of the light sensitive proteins being used. | ||
<!-- There are many assumptions about the action of the light sensitive protein in this calculation and they need addressing --> | <!-- There are many assumptions about the action of the light sensitive protein in this calculation and they need addressing --> | ||
| - | |||
| - | |||
| - | + | ==== Biological pathway simulation ==== | |
| - | + | Our stochastic model is comprised of red, green and a blue light activated pathways. Using multiple runs of the simulation, the stability of the tri-chromatic control system, its pigment balance, and image development time can all be tested in-silico. These have implications for future projects that might use a similar system and the model provides a platform for others to build on in the future. | |
| - | Each | + | Each pathway is independent in their signalling but shares the common resources of the cell. The independent variable is the activation rate of each light sensor species, this is determined by the incident light and effects the expression of pigments, the dependant variable. |
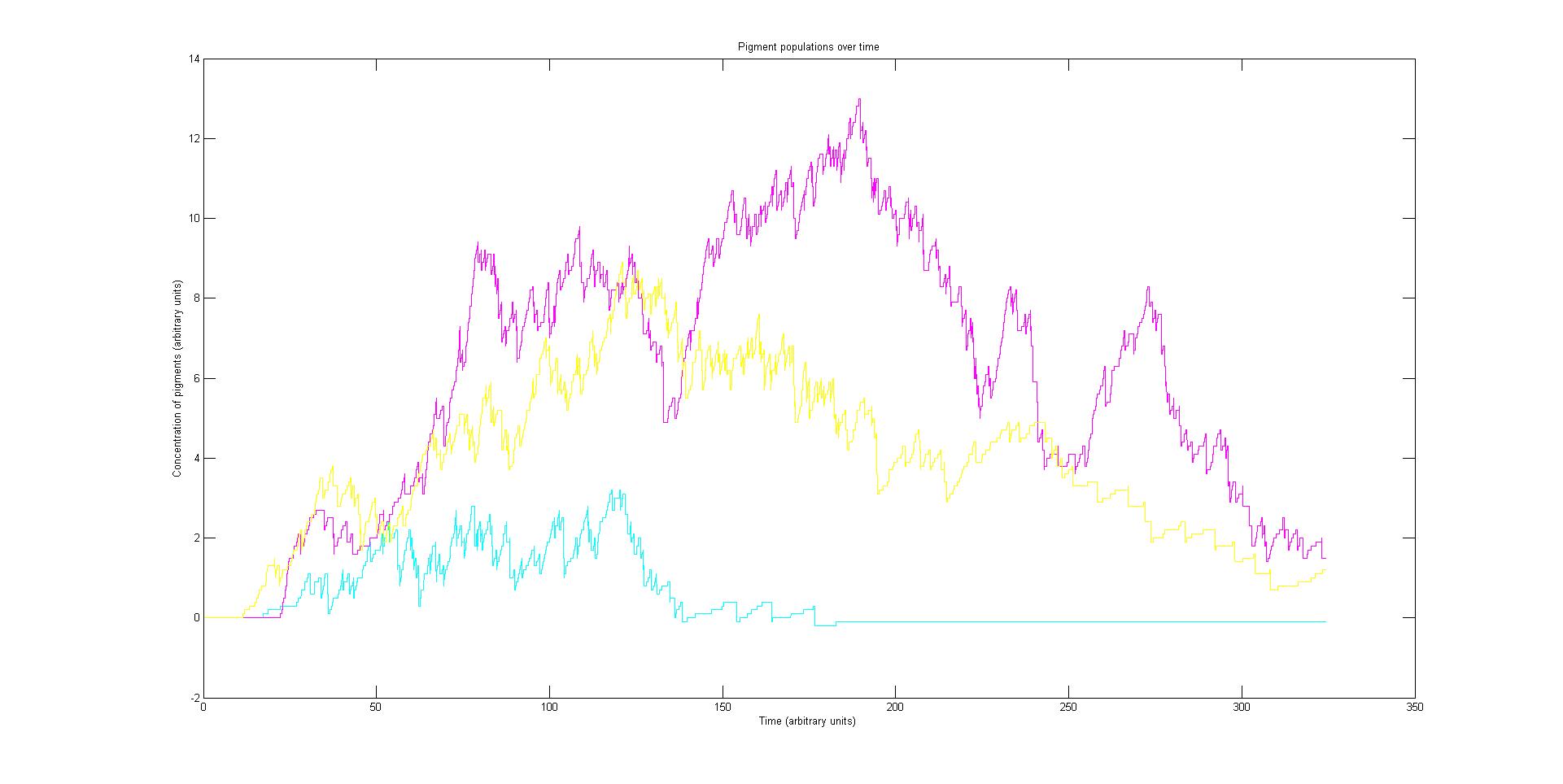
| - | + | [[Image:Alpha1.jpg|thumb|center|upright=5.0| This image is an alpha run of our stochastic model. It shows the populations of the cyan, magenta and yellow pigments over time in a single cell. In this simulation all three light sensor had the same activation rate]] | |
| - | The | + | The Kappa model describes in detail the pathway specific biochemistry and includes simplified descriptions of pathway related biochemistry. For example, the controlled transcription and translation of pigment proteins is described in full, but the constitutive transcription and translation of light sensor proteins is simplified. The rates were created to recreate experimental results recorded in literature<!-- CITATION NEEDED -->. With experimental results this model can be readily updated to accurately describe our system. |
| - | It | + | All molecule species, with the exception DNA, are constitutively expressed. Molecule species apart from DNA, RNA polymerase and ribosomes have spontaneous decay rates, as variance during the time period of the system run has been taken as negligible. Phosphorylated species spontaneously dephosphorylation rate. Molecule species are represented as agents with the exceptions of the pigment proteins and ATP, which are modelled as concentrations of tokens. |
| + | |||
| + | The initial condition only contains DNA, RNA polymerase and ribosomes, and OmpR (which is naturally present in the E.coli), all other agents and tokens are produced by processes of transcription and translation. Constitutive expression of light sensors and intermediates are required before the cell can begin to respond to light. | ||
| + | |||
| + | ==== Cell absorption spectrum calculation ==== | ||
| + | |||
| + | The final output of our model is a surface plot of absorption intensity as a function of time and wavelength. It reveals how the absorption spectrum and thus the colour of the cell changes with time. It also reveals how long it takes to reach equilibrium, the moment of greatest contrast and many other important features. | ||
| + | |||
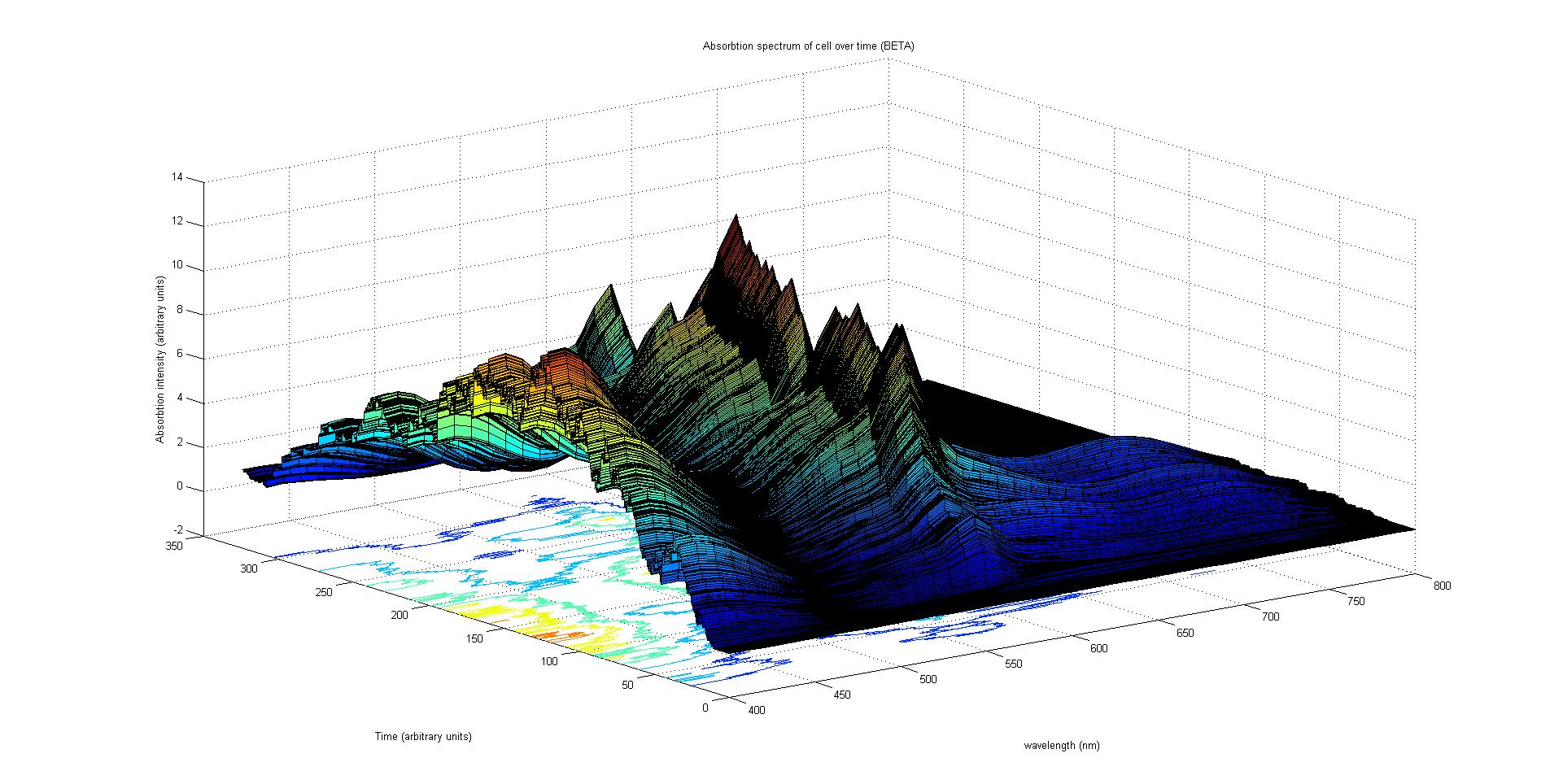
| + | [[Image:Beta2.jpg|thumb|center|upright=5.0| This image shows the combined absorption spectra of the cyan, magenta and yellow pigments over time calculated from the data in the graph alhpa1 above. It is a beta run of our absorption spectrum plot (MATLAB).]] | ||
| + | |||
| + | Each protein species has an associated absorption spectrum. | ||
| + | <center><TABLE cellpadding="20"> | ||
| + | <TR> | ||
| + | <TD> [[Image:PigmentSpectra1.jpg|thumb|center|upright=5.0| This image shows the absorption spectra of the cyan, magenta and yellow pigment proteins]] | ||
| + | <TD> [[Image:SensorSpectra.jpg|thumb|center|upright=5.0| This image shows the absorption spectra of the red (Cph8), green (CcaS) and blue (LOVtap) sensor proteins. Note we are using YF1 instead of LOVtap but the LOVtap spectrum is used in our model.]] | ||
| + | </TR> | ||
| + | </TABLE></center> | ||
| + | |||
| + | This multiplied by the population of the protein at a point in time gives the contribution of that protein to the total absorption spectrum of the cell at that moment. The sum of the contributions from each protein plus the absorption spectrum of the background cell gives the total absorption spectrum of the cell at that moment. This calculation repeated for every time step gives the total absorption of the cell over time. | ||
| + | |||
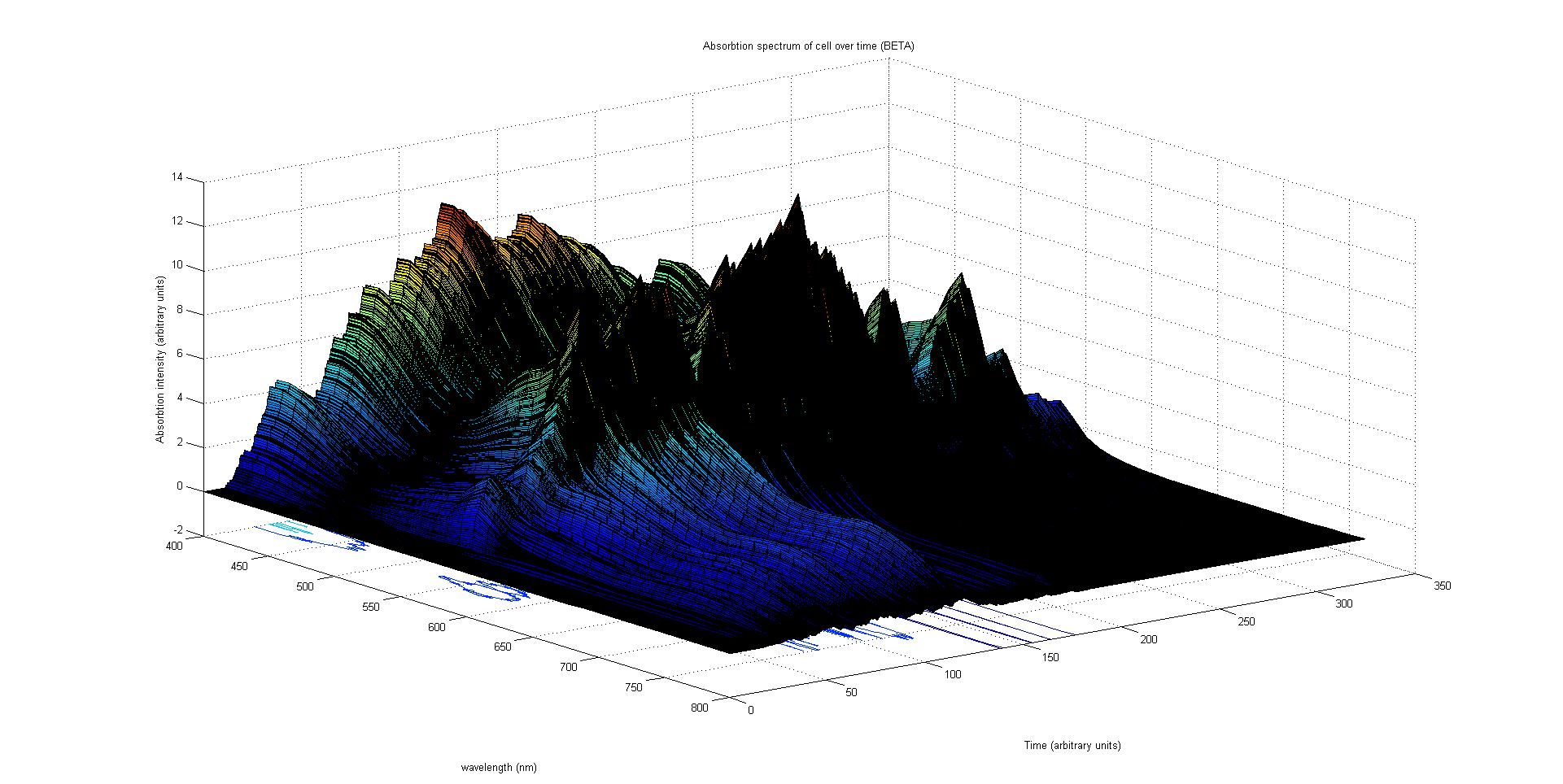
| + | [[Image:Beta2a.jpg|thumb|center|upright=5.0| This images show the combined absorption spectra of the cyan, magenta and yellow pigments over time calculated from the data in the graph alhpa1 above. This is the same surface as in the previous plot but viewed from another direction (90 degrees anticlockwise from the previous image)]] | ||
<!-- <gallery caption="Pathway block diagrams" perrow=6 widths=275 heights=300> | <!-- <gallery caption="Pathway block diagrams" perrow=6 widths=275 heights=300> | ||
| Line 71: | Line 131: | ||
--> | --> | ||
| - | == | + | ==== Data sources ==== |
| - | + | The model is based on data from literature and theoretical conjecture: | |
| + | * The stochastic rates are ballpark figures for <i>E.coli</i> cells and are non specific. | ||
| + | * All absorbtion spectra do not have relative intensity all peak around 1 arbitrary unit. | ||
| + | * The light sensor absorption spectra are estimates of data taken from literature. | ||
| + | * The pigment sensor absorption spectra are estimates of the pigment absoption of colour printer ink. | ||
| + | * The operation of the light sensor is an idealisation of the true relationship between sensor activation and incident light | ||
| - | + | These will all contribute to the errors in our model but have been chosen to demonstrate our model in action. These can easily be replace with experimental results. | |
| - | + | ==== Results and analysis ==== | |
| - | + | All results in this section are obtained from the beta version of our model. In this section we will discuss the results that our model has produced and also how sound the model's operation is. | |
| - | + | ==== Results of model ==== | |
| - | + | Preliminary results suggest that the mix of colours in any individual <i>E.coli</i> system is very unpredictable. The model was run multiple times with all sensors at the same activation rate. The mix of colours between separate simulations is very unstable. However the average for a cohort of cell systems is more stable. It is due to the relatively small number of DNA agents with which the RNAP interacts. This amplifies the effects of chance and causes the instability observed in the model. | |
| - | This | + | [[Image:Multi-run.png|thumb|center|upright=5| This shows the pigment populations during 5 runs of a cell given the same initial conditions each time. It demonstrates the instability in the gene expression and also inequality in the magnitude of expression. The 'x' character lines represent the average of the 5 runs and are more stable as a results.]] |
| - | + | This will serve reduce the spacial control of tri-chromatic control system in <i>E.coli</i> as a predictable average expression of a gene will require multiple individuals. For a biocamera this will reduce the pixel density and therefore the resolution. One solution to this potential problem could be to introduce many copies of the light regulated genes. This could also serve to prevent one pathway dominating by having greater numbers of less prolific light regulated genes to compensate. | |
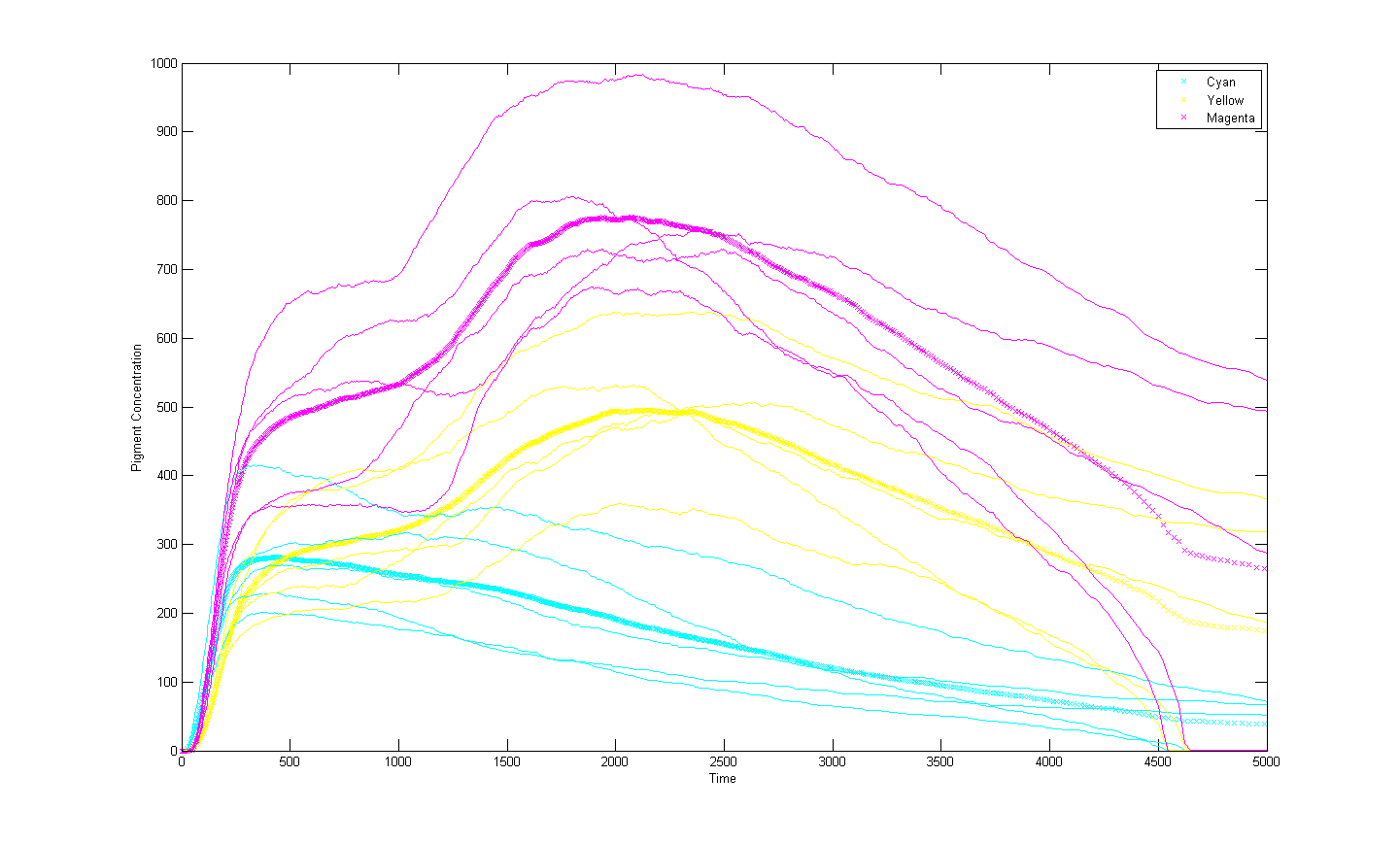
| - | This | + | [[Image:Multi-run2.png|thumb|center|upright=5| This shows the pigment populations during 5 runs of a cell given the same initial conditions as the previous graph. However in this run there is a ten fold increase in the number of pathway DNA present in the system. It demonstrates increased stability as a results and this supports the idea that the low number of DNA in a cell amplifies the effect of chance in the simulation. As before 'x' character lines represent the average of the 5 runs and are more stable as a results.]] |
| - | == Bibliography == | + | An increase in light regulated DNA has been shown to stabilise the system. However it is impractical and expensive to insert large amounts of DNA into individual bacteria with current methods. |
| + | |||
| + | ==== User experience ==== | ||
| + | |||
| + | The activation rate script is simple and deterministic. The results for activation rates are usually very high but that can be counterbalanced by either reducing the intensity of either the sensor absorption spectrum and/or the incident light. The results of this method have two important parrallels with the physical reality making it a working simplified model of sensor activation. | ||
| + | |||
| + | The Stochastic model is very sensitive to changes in rates and initial conditions. With very different behaviour resulting from only small pertubations. This is due to the stochastic nature of the model compounded by the small number of DNA agents as discussed before. | ||
| + | |||
| + | The absorption spectrum over time surface plot has to potential to produce accurate results. We have writen a script in MATLAB® that calculates the reflected light over time given the absorption over time and the incident light. It is possible to write a program that converts the reflected light spectrum over time into a colour over time. Regrettfuly we did not have the time to attempt such an edevour. | ||
| + | |||
| + | ==== Sources of error ==== | ||
| + | |||
| + | The model we have created is limited by the lack of experimental data to test and update it. As a result the data used in our model has a variety of sources and these indroduce many sources of error. | ||
| + | |||
| + | Firstly the rules describing the biochemistry of our cell addressed only the reactions unique to our pathway in detail and related reactions in a simplified manner. A small number of external conditions were addressed such as the competition for RNA polymerase and ribosomes between our pathway and other activity in the cell. This however means that the model does not take into account any interference from other cell activity. This is a source of error in our pathway simulation, however it is unlikely to the primary source of error. | ||
| + | |||
| + | Secondly the absorbtion spectra for all proteins and the background cell do not have their relative absorbtion intensities. Furthermore the spectra of the light sensors are approximations to data in literature, but the blue light sensor's absorbtion is that of LOVtap not YF1. LOVtap and YF1 are both light oxygen voltage blue light receptors and therefore we expect them to have a similar absorbtion spectrum. The spectra of the pigments are approximations of the standard cyan, magenta and yellow pigments used in colour printers. Therefore the pigment absorbtion spectra only represent the ideal colour of our pigments. This final point does not affect our rule-based stochastic simulation. The inaccuracy in our spectra causes an uncertainty in the calculation of activation rate and a greater uncertainty in the calculation of the cell's absorbtion. | ||
| + | |||
| + | Thirdly the nature of the activation of the light sensors is unknown and the method we use to calculate the activation rate is likely to be errenous. This introduces another source of error in the calculation of sensor activation rates. | ||
| + | |||
| + | Fourthly the rates in our rule-based model are ballpark figures taken from literature and are not specific to the reactions in our model. This is the primary source of error in our pathway simulation. | ||
| + | |||
| + | === Conclusions === | ||
| + | |||
| + | <!-- This section will contain our conclusions about the model --> | ||
| + | |||
| + | The multitude and magnitude of error sources in our model mean that no accurate conclusions about our synthetic biological system can be drawn from it. | ||
| + | |||
| + | However our preliminary results suggest that tri-chromatic control of gene expression in <i>E.coli</i> is very unstable for individual cells. This may be due to the small number of DNA blocks in our system amplifying the effect of chance. For large groups of <i>E.coli</i> the average expression is more predictable. This is to be expected in a stochastic system but the degree to which expression varies between individuals under identical circumstances surprised us. This suggests that instability will reduce the spatial control that our tri-chromatic system can have. It is possible that increasing the number of light regulated genes may reduce the instability and increase the potential spatial resolution. | ||
| + | |||
| + | The model itself is a good foundation for future multi-chromatic control modelling efforts. The data that feeds the model can easily be replace with experimental data. The kappa file is well layed out and comprehensible with some understanding of kappa syntax. We think that it has the potential to provide a useful tool for modelling similar systems or as a resource for others to build rule-based models of their own. | ||
| + | |||
| + | === Future === | ||
| + | |||
| + | <!-- This section will contain our opinions about the future of the model --> | ||
| + | |||
| + | Our model serves as a framework for modelling tri-chromatic control in <i>E.coli</i>. The foremost action to be taken in the future is the successful synthesis and testing of a working tri-chromatic control system. The experimental results could be used to update and test the model. | ||
| + | |||
| + | Improvements to the workings of the model include allowing the input signal to change with time. This means updating the rates in the kappa model while the model is being run by KaSiM. This requires either greater integration between KaSiM and mathematics programs like MATLAB or a more powerful mathematics suite in KaSiM. It is possible also to write a program the converts the cell's absorbtion spectrum over time into its colour over time. This is something that we did not have time to do. | ||
| + | |||
| + | Increasing the number of light regulated DNA in a cell reduces the instability. Perhaps in the future it will become affordable to genome integrate large numbers of light regulated genes to provide reliable and extremely high spatial control of bacteria using mulitple wavelengths. | ||
| + | |||
| + | === Bibliography === | ||
<!-- | <!-- | ||
| Line 98: | Line 205: | ||
From dusk till dawn: one-plasmid systems for light-regulated gene expression. | From dusk till dawn: one-plasmid systems for light-regulated gene expression. | ||
| + | |||
| + | http://www.cis.rit.edu/research/thesis/bs/1998/hoffman/Thesis.html | ||
| + | |||
| + | Edinburgh 2010, 2011 | ||
| + | |||
| + | Kasim manual | ||
| + | |||
| + | 'Modular Modelling in Synthetic Biology: Light-Based Communication in E. coli' Stewart and Kanamori | ||
--> | --> | ||
| + | |||
| + | Edinburgh iGEM 2010 | ||
| + | https://2010.igem.org/Team:Edinburgh/ | ||
| + | |||
| + | |||
| + | |||
| + | KaSim3 reference manual | ||
| + | (release 3.4) | ||
| + | Jérôme Feret and Jean Krivine1 | ||
| + | KappaLanguage.org | ||
| + | |||
| + | |||
| + | |||
| + | J Mol Biol. 2011 Jan 14;405(2):315-24. doi: 10.1016/j.jmb.2010.10.038. Epub 2010 Oct 28. | ||
| + | Multichromatic control of gene expression in Escherichia coli. | ||
| + | Tabor JJ, Levskaya A, Voigt CA. | ||
| + | |||
| + | Department of Pharmaceutical Chemistry, University of California, San Francisco, CA 94158, USA. | ||
| + | |||
| + | |||
| + | |||
| + | J Mol Biol. 2012 Mar 2;416(4):534-42. doi: 10.1016/j.jmb.2012.01.001. Epub 2012 Jan 8. | ||
| + | From dusk till dawn: one-plasmid systems for light-regulated gene expression. | ||
| + | Ohlendorf R, Vidavski RR, Eldar A, Moffat K, Möglich A. | ||
| + | Source | ||
| + | |||
| + | Humboldt-Universität zu Berlin, Institut für Biologie, Biophysikalische Chemie, Invalidenstraße 42, 10115 Berlin, Germany. | ||
| + | |||
| + | |||
| + | |||
| + | 277, 27 October 2011, Pages 77–87 | ||
| + | |||
| + | Modular Modelling in Synthetic Biology: Light-Based Communication in E. coli ☆ | ||
| + | The Second International Workshop on Interactions between Computer Science and BiologyDonal Stewart E-mail the corresponding author | ||
| + | DemonSoft Ltd, Edinburgh, United Kingdom | ||
| + | John Roger Wilson-Kanamori E-mail the corresponding author | ||
| + | |||
| + | School of Informatics, University of Edinburgh, Edinburgh, United Kingdom | ||
| + | |||
| + | |||
| + | |||
| + | Applications of the Kubelka-Munk Color Model to Xerographic Images | ||
| + | Final Report | ||
| + | Kristen Hoffman | ||
| + | Center for Imaging Science | ||
| + | Rochester Institute of Technology | ||
| + | May 1998 | ||
| + | |||
| + | |||
| Line 120: | Line 284: | ||
# We shall construct rudimentary equations for each step in our pathways. | # We shall construct rudimentary equations for each step in our pathways. | ||
# Throughout the project our model will be updated either in light of experimental results or new ideas. --> | # Throughout the project our model will be updated either in light of experimental results or new ideas. --> | ||
| + | |||
| + | |||
| + | |||
| + | |||
Latest revision as of 11:18, 15 October 2013
Modelling
Abstract
We have developed a stochastic model of a colour bio-camera system in E.coli. The model takes the light incident of the bacteria and stochastically estimates the light regulated expression of pigment proteins over time. The population of proteins over time is combined with their absorption spectra and plotted as a surface showing the cell’s absorption spectrum over time. There have been insufficient experimental results to test the model with rates and absorption spectra taken from literature and theoretical conjecture. This prevents us from drawing accurate conclusions. However our preliminary results suggest that a collection of cells will show a reliable colour but with a very high variance between individuals. This reduces the spatial control of expression and thus the resolution of any bio-camera. One way to counter this problem and the problem of one pathway dominating the others is to insert multiple copies of the light regulated genes. This causes spatial control to be increased as the behaviour of each individual is more predictable. This model requires experimental data to test it and update it to produce an accurate description of our biological system. In its current for it provides a well documented framework for future rule-based modelling of similar systems.
Introduction
Our model predicts how the absorption spectrum of a modified E. coli changes over time in response to a given incident light spectrum. It can estimate key properties of the system such as stability, development time of the image, and the balance of colours.
The model is split into three sections. Initially, the reaction of each sensor species to a given incident light spectrum is calculated. This information feeds to the heart of the model, which involves simulating the biochemistry of our designed pathways. Finally the data from the simulation is used to plot the absorption spectrum of the system as it varies with time.
Currently the model is based on results from literature and theoretical conjecture as sufficient experimental results are not available. The motivation for the model is to numerically characterize our bio-bricks for future use and to help us create the first colour coliroid. With experimental results the model could readily be updated to fulfill its original mandate.
Control of bacteria using light has many applications. Di-chromatic control of bacteria has been achieved, the challenge now is for Tri-chromatic control. Our lab project aims to produce a full colour ‘coliroid’ to demonstrate tri-chromatic control of bacteria. The modified E.coli are designed to carry three independent light sensitive pathways that each control the production of a specific pigment protein.
A reliable computer model is essential for accurate implementation of tri-chromatic control. In the case of the theoretical bio-camera it is required to achieve an even balance of colours and optimum development time. In other applications it is essential to have accurate temporal control over the expression of light regulated genes.
The model is based around KaSim, a stochastic simulator that reads Kappa, a rule based language. Stochastic simulation has the advantage over deterministic simulation because it replicates the random nature of brownian motion in cells more accurately. Rule based languages are particularly useful for biochemical signalling as the physical reactions are readily converted into rules. Matlab is used to process the signal from the incident light to the KaSim simulation. The outputs of the simulation is used in MATLAB to calculate the change in absorbtion spectrum over time.
Modelling Software
The modelling was primarily conducted using two platforms; [http://www.mathworks.co.uk/products/matlab/ MATLAB®] and KaSiM. Together they provide the necessary tools to create an accurate model of our system.
They have been used succesfully by previous iGEM teams, notably the Edinburgh 2010 and 2011 teams, both of which one Best Model at the European Jamboree. This was the primary reason we chose the Kappa language to write our model in.
KaSiM is a [http://en.wikipedia.org/wiki/Stochastic stochastic] simulator that executes files written in [http://www.kappalanguage.org/ Kappa]. Kappa is a rule based modelling language for protein interaction networks. To download KaSiM or the manual please visit the KaSiM page.
KaSiM
KaSiMis a [http://en.wikipedia.org/wiki/Stochastic stochastic]simulator of rule based models written in the Kappa language. It is open source program that uses a variation of the Gillespie's algorithm to generate statistically correct trajectories. We advise you take the time to read the introduction to KaSiM in the KaSiM manual .
Kappa
Kappa is a high level rule-based language designed to describe protein interaction networks. Rule-based languages describe a system by a governing set of rules. There is no change in the configuration of the system without the execution of a rule. There are five fundamental parts to the kappa language; agents, tokens, rules, rates and observables. A quick introduction to these concepts is given below. For more detailed information we suggest visiting the [http://www.kappalanguage.org/ Kappa] website or for full examples [http://rulebase.org/ RuleBase] which offers a repository of complete rule based models. We can also recommend the 2012 Edinburgh iGEM team's Introduction to Kappa which proved useful to us early on.
Agents and tokens
Agents and tokens represent the constituent molecules of a system. Agents are quantified in terms of their population number and are characterised having binding sites. Whereas tokens are quantified in terms of their concentration and do not have binding sites.
Rules
Rules represent specific bio-chemical processes, and involve either tokens, agents or both. Each rule has an initial condition which must be satisfied for the rule to be valid. If the conditions are met, the rule is applied according to the rate.
Rates
Each rule has a specific rate constant which determines the number of times the rule is applied for each instance of the rule.
Observables
Observables determine the output data in terms of either agent count or token concentration.
MATLAB®
[http://www.mathworks.co.uk/products/matlab/ MATLAB®] is a high-level language and interactive environment for numerical computation, visualization, and programming. It can be used to analyse data, develop algorithms, and create models and applications. For guidance on using MATLAB® please see the [http://www.mathworks.co.uk/help/matlab/ mathworks documentation] .
The model
The model describes the absorption spectrum of the cell as a function of time and a given time invariant incident light spectrum. This is achieved by calculating the effect of the known light input on the individual light sensitive proteins. This information is then fed into a stochastic simulation of the biological pathways that computes how the population of sensors and pigments vary over time. The total absorption spectrum over time is given by the sum of the product of every protein's population and the corresponding absorption spectrum of the protein. In this section each of these steps in considered in the following subsections.
It will be helpful to read the theory page and the modelling software section of this page before reading further as an understanding of the biological pathways and software is important.
Sensor activation rate calculation
In the rule based model each sensor is thought of as a two-state binary system (on/off). In the on state it catalyses the phosphorylation of the primary intermediate, sending a signal to the downstream pathway. In the off state it does not.
Activation by light either turns a sensor on or off depending on the sensor species; red and blue light sensors are switched off when activated, while the green light sensor is switched on when activated. The activation rate is the forward rate of the rule that governs the state of a sensor. Its counterpart, the deactivation rate is the backward rate of the same rule and is constant. The activation rate is calculated as the integral over all space of the product of the sensor's frequency response with the frequency spectrum of the incident light.
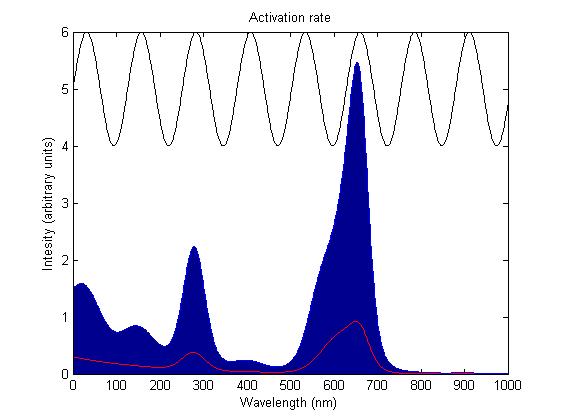
This has two important implications. Firstly it means that the sensor will not be activated by light that has no overlap between its spectrum and the frequency response of the sensor. Secondly there will be a saturation point at high intensity light where increasing the intensity will not appreciably affect the system as the activation rate will dominate the deactivation rate. These characteristics are representative of those of the light sensitive proteins being used.
Biological pathway simulation
Our stochastic model is comprised of red, green and a blue light activated pathways. Using multiple runs of the simulation, the stability of the tri-chromatic control system, its pigment balance, and image development time can all be tested in-silico. These have implications for future projects that might use a similar system and the model provides a platform for others to build on in the future.
Each pathway is independent in their signalling but shares the common resources of the cell. The independent variable is the activation rate of each light sensor species, this is determined by the incident light and effects the expression of pigments, the dependant variable.
The Kappa model describes in detail the pathway specific biochemistry and includes simplified descriptions of pathway related biochemistry. For example, the controlled transcription and translation of pigment proteins is described in full, but the constitutive transcription and translation of light sensor proteins is simplified. The rates were created to recreate experimental results recorded in literature. With experimental results this model can be readily updated to accurately describe our system.
All molecule species, with the exception DNA, are constitutively expressed. Molecule species apart from DNA, RNA polymerase and ribosomes have spontaneous decay rates, as variance during the time period of the system run has been taken as negligible. Phosphorylated species spontaneously dephosphorylation rate. Molecule species are represented as agents with the exceptions of the pigment proteins and ATP, which are modelled as concentrations of tokens.
The initial condition only contains DNA, RNA polymerase and ribosomes, and OmpR (which is naturally present in the E.coli), all other agents and tokens are produced by processes of transcription and translation. Constitutive expression of light sensors and intermediates are required before the cell can begin to respond to light.
Cell absorption spectrum calculation
The final output of our model is a surface plot of absorption intensity as a function of time and wavelength. It reveals how the absorption spectrum and thus the colour of the cell changes with time. It also reveals how long it takes to reach equilibrium, the moment of greatest contrast and many other important features.
Each protein species has an associated absorption spectrum.
This multiplied by the population of the protein at a point in time gives the contribution of that protein to the total absorption spectrum of the cell at that moment. The sum of the contributions from each protein plus the absorption spectrum of the background cell gives the total absorption spectrum of the cell at that moment. This calculation repeated for every time step gives the total absorption of the cell over time.
Data sources
The model is based on data from literature and theoretical conjecture:
- The stochastic rates are ballpark figures for E.coli cells and are non specific.
- All absorbtion spectra do not have relative intensity all peak around 1 arbitrary unit.
- The light sensor absorption spectra are estimates of data taken from literature.
- The pigment sensor absorption spectra are estimates of the pigment absoption of colour printer ink.
- The operation of the light sensor is an idealisation of the true relationship between sensor activation and incident light
These will all contribute to the errors in our model but have been chosen to demonstrate our model in action. These can easily be replace with experimental results.
Results and analysis
All results in this section are obtained from the beta version of our model. In this section we will discuss the results that our model has produced and also how sound the model's operation is.
Results of model
Preliminary results suggest that the mix of colours in any individual E.coli system is very unpredictable. The model was run multiple times with all sensors at the same activation rate. The mix of colours between separate simulations is very unstable. However the average for a cohort of cell systems is more stable. It is due to the relatively small number of DNA agents with which the RNAP interacts. This amplifies the effects of chance and causes the instability observed in the model.

This will serve reduce the spacial control of tri-chromatic control system in E.coli as a predictable average expression of a gene will require multiple individuals. For a biocamera this will reduce the pixel density and therefore the resolution. One solution to this potential problem could be to introduce many copies of the light regulated genes. This could also serve to prevent one pathway dominating by having greater numbers of less prolific light regulated genes to compensate.
An increase in light regulated DNA has been shown to stabilise the system. However it is impractical and expensive to insert large amounts of DNA into individual bacteria with current methods.
User experience
The activation rate script is simple and deterministic. The results for activation rates are usually very high but that can be counterbalanced by either reducing the intensity of either the sensor absorption spectrum and/or the incident light. The results of this method have two important parrallels with the physical reality making it a working simplified model of sensor activation.
The Stochastic model is very sensitive to changes in rates and initial conditions. With very different behaviour resulting from only small pertubations. This is due to the stochastic nature of the model compounded by the small number of DNA agents as discussed before.
The absorption spectrum over time surface plot has to potential to produce accurate results. We have writen a script in MATLAB® that calculates the reflected light over time given the absorption over time and the incident light. It is possible to write a program that converts the reflected light spectrum over time into a colour over time. Regrettfuly we did not have the time to attempt such an edevour.
Sources of error
The model we have created is limited by the lack of experimental data to test and update it. As a result the data used in our model has a variety of sources and these indroduce many sources of error.
Firstly the rules describing the biochemistry of our cell addressed only the reactions unique to our pathway in detail and related reactions in a simplified manner. A small number of external conditions were addressed such as the competition for RNA polymerase and ribosomes between our pathway and other activity in the cell. This however means that the model does not take into account any interference from other cell activity. This is a source of error in our pathway simulation, however it is unlikely to the primary source of error.
Secondly the absorbtion spectra for all proteins and the background cell do not have their relative absorbtion intensities. Furthermore the spectra of the light sensors are approximations to data in literature, but the blue light sensor's absorbtion is that of LOVtap not YF1. LOVtap and YF1 are both light oxygen voltage blue light receptors and therefore we expect them to have a similar absorbtion spectrum. The spectra of the pigments are approximations of the standard cyan, magenta and yellow pigments used in colour printers. Therefore the pigment absorbtion spectra only represent the ideal colour of our pigments. This final point does not affect our rule-based stochastic simulation. The inaccuracy in our spectra causes an uncertainty in the calculation of activation rate and a greater uncertainty in the calculation of the cell's absorbtion.
Thirdly the nature of the activation of the light sensors is unknown and the method we use to calculate the activation rate is likely to be errenous. This introduces another source of error in the calculation of sensor activation rates.
Fourthly the rates in our rule-based model are ballpark figures taken from literature and are not specific to the reactions in our model. This is the primary source of error in our pathway simulation.
Conclusions
The multitude and magnitude of error sources in our model mean that no accurate conclusions about our synthetic biological system can be drawn from it.
However our preliminary results suggest that tri-chromatic control of gene expression in E.coli is very unstable for individual cells. This may be due to the small number of DNA blocks in our system amplifying the effect of chance. For large groups of E.coli the average expression is more predictable. This is to be expected in a stochastic system but the degree to which expression varies between individuals under identical circumstances surprised us. This suggests that instability will reduce the spatial control that our tri-chromatic system can have. It is possible that increasing the number of light regulated genes may reduce the instability and increase the potential spatial resolution.
The model itself is a good foundation for future multi-chromatic control modelling efforts. The data that feeds the model can easily be replace with experimental data. The kappa file is well layed out and comprehensible with some understanding of kappa syntax. We think that it has the potential to provide a useful tool for modelling similar systems or as a resource for others to build rule-based models of their own.
Future
Our model serves as a framework for modelling tri-chromatic control in E.coli. The foremost action to be taken in the future is the successful synthesis and testing of a working tri-chromatic control system. The experimental results could be used to update and test the model.
Improvements to the workings of the model include allowing the input signal to change with time. This means updating the rates in the kappa model while the model is being run by KaSiM. This requires either greater integration between KaSiM and mathematics programs like MATLAB or a more powerful mathematics suite in KaSiM. It is possible also to write a program the converts the cell's absorbtion spectrum over time into its colour over time. This is something that we did not have time to do.
Increasing the number of light regulated DNA in a cell reduces the instability. Perhaps in the future it will become affordable to genome integrate large numbers of light regulated genes to provide reliable and extremely high spatial control of bacteria using mulitple wavelengths.
Bibliography
Edinburgh iGEM 2010 https://2010.igem.org/Team:Edinburgh/
KaSim3 reference manual (release 3.4) Jérôme Feret and Jean Krivine1 KappaLanguage.org
J Mol Biol. 2011 Jan 14;405(2):315-24. doi: 10.1016/j.jmb.2010.10.038. Epub 2010 Oct 28. Multichromatic control of gene expression in Escherichia coli. Tabor JJ, Levskaya A, Voigt CA.
Department of Pharmaceutical Chemistry, University of California, San Francisco, CA 94158, USA.
J Mol Biol. 2012 Mar 2;416(4):534-42. doi: 10.1016/j.jmb.2012.01.001. Epub 2012 Jan 8. From dusk till dawn: one-plasmid systems for light-regulated gene expression. Ohlendorf R, Vidavski RR, Eldar A, Moffat K, Möglich A. Source
Humboldt-Universität zu Berlin, Institut für Biologie, Biophysikalische Chemie, Invalidenstraße 42, 10115 Berlin, Germany.
277, 27 October 2011, Pages 77–87
Modular Modelling in Synthetic Biology: Light-Based Communication in E. coli ☆ The Second International Workshop on Interactions between Computer Science and BiologyDonal Stewart E-mail the corresponding author DemonSoft Ltd, Edinburgh, United Kingdom John Roger Wilson-Kanamori E-mail the corresponding author
School of Informatics, University of Edinburgh, Edinburgh, United Kingdom
Applications of the Kubelka-Munk Color Model to Xerographic Images Final Report Kristen Hoffman Center for Imaging Science Rochester Institute of Technology May 1998
 "
"