Team:Heidelberg/Delftibactin/Delftibactin
From 2013.igem.org
| (9 intermediate revisions not shown) | |||
| Line 17: | Line 17: | ||
<div style="margin-top:10%;"> | <div style="margin-top:10%;"> | ||
<h1><span style="font-size:180%;color:#FFCC00;">Delftibactin.</span><span class="text-muted" style="font-size:120%"> Bringing it all together.</span></h1> | <h1><span style="font-size:180%;color:#FFCC00;">Delftibactin.</span><span class="text-muted" style="font-size:120%"> Bringing it all together.</span></h1> | ||
| - | <p style="font-size:14px"> | + | <p style="font-size:14px">Let's explore the vision to recycle gold from electronic waste using recombinantly expressed delftibactin.</p> |
</div> | </div> | ||
<div class="row"> | <div class="row"> | ||
| Line 140: | Line 140: | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<div class="methods jumbotron" data-spy="scroll" data-target="#navbarExample" data-offset="0" style="height:364px"> | <div class="methods jumbotron" data-spy="scroll" data-target="#navbarExample" data-offset="0" style="height:364px"> | ||
| - | < | + | <h3>Graphical Abstract</h3> |
| - | < | + | <div style="height:200px;"> |
| - | + | <a class="fancybox fancyGraphical" rel="group" href="https://static.igem.org/mediawiki/2013/e/e3/Heidelberg_ga_delf.png"> | |
| + | |||
| + | <img style="height:200px; margin-bottom:10px; padding:1%;border-style:solid;border-width:1px;border-radius: 4px; border-color: grey;" src="https://static.igem.org/mediawiki/2013/e/e3/Heidelberg_ga_delf.png"></img> | ||
| + | |||
| + | </a> | ||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| - | |||
| - | |||
<div class="row"> | <div class="row"> | ||
| - | |||
<!--Start Weekly Labjournal--> | <!--Start Weekly Labjournal--> | ||
| Line 267: | Line 268: | ||
</div> | </div> | ||
</div> | </div> | ||
| - | |||
<!--Week20--> | <!--Week20--> | ||
| Line 318: | Line 318: | ||
</div> | </div> | ||
</div> | </div> | ||
| - | |||
<!--Week22--> | <!--Week22--> | ||
Latest revision as of 02:19, 5 October 2013


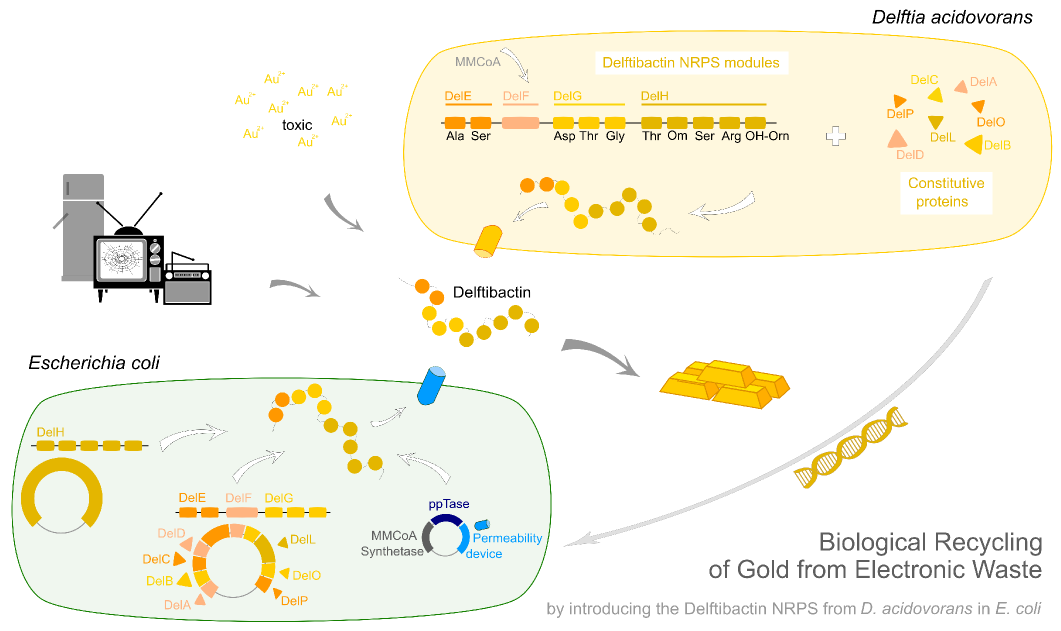
Delftibactin. Bringing it all together.
Let's explore the vision to recycle gold from electronic waste using recombinantly expressed delftibactin.
Week 1
We obtained D. acidovorans DSM-39 from the DSMZ and successfully reproduced the paper of Johnsson et al. D. acidovorans is capable to precipitate solid gold from gold chloride solution as purple-black nanoparticles. Already at low concentrations of gold chloride, gold nonaparticles are precipitated increasing with concentration of gold chloride in solution. In our experiments, precipitation on agar plates worked even better than described in the paper.
Week 3
We were able to dissolve gold-containing parts of an old CPU and established a protocol for recovery of gold as soluble gold salts from electronic waste. Moreover, we were able to show specific precipitation of solid gold by D. acidovorans from this "dissolved electronic waste".
Week 15
Using supernatants from the new Delftia acidovorans strain SPH-1, we showed precipitation of gold chloride solution to gold nanoparticles. Furthermore, we melted the purple-black nanoparticles to shiny solid gold.
Week 19
We optimized growth conditions of D. acidovorans and evaluated endogenous background precipitation of metall ions in the possible target E. coli strains E. coli DH10ß and BL21 DE3 following incubation over night on LB and ACM plates. Delftia acidovorans did not exceed E. coli, most probably due to insufficient cultivation time. When grown on LB plates, neither D. acidovorans nor E. coli showed any reactivity.
Week 20
We continued optimized growth conditions of D. acidovorans and testing various E. coli strains for their endogenous capability to precipitate gold in order to identify the strain with the least background to be used as target strain after 2 and 3 days as well as grown at 30°C and room temperature. A cultivation at 30°C for 3 days was identified as optimal. We also started to establish purification of Delftibactin using HP20 resins and successfully verified presence of Delftibactin in the supernatant of D. acidovorans SHP-1. Additionally, we proved precipitation of gold by the purified Delftibactin and detected it by Micro-TOF File:20130911Malditof.pdf. Moreover, we triple-electroporated the final DelRest construct, the final MMCoA plasmid and the first promissing DelH clone into E. coli DH10ß. The Micro-TOF has to be repeated again next week.
Week 21
We repeated last week's Micro-TOF of the first promossing triple-clone, which did not show detectable expression of Delftibactin.
Week 22
Possible E. coli target strains BL21 DE, DH10ß and NEB Turbo were analyzed for their background expression and indcibility. E. coli BL21 DE was identified as best of these three. It was electroporated with DelRest and pIK8.6 and of these, electrocompetent cells were prepared.
Week 23
E. coli BL21 DE + DelRest + pIK8.6 were elctroporated with the DelH clone C5, which harbors a amino acid substitution at the beginning of DelH. Its capability to precipitate gold from solution was analyzed on ACM plates following induction by IPTG. Due to a contamination, the results were inconclusive. The production of Delftibactin was accessed by Micro-TOF. The results were inconclusive, since the ''D. acidovorans'' positive control was negative, the experiment has to be repeated.
29-04 - 05-05-13
Reproduction of Johnsson et al. Paper
Aim
Reproduction of Johnsson et al. paper: Precipitating gold with D.acidovorans on agar plate, in culture, the medium of the culture and possibly purified delftibactin.
Preparations
- Obtain D. acidovorans from DSMZ
- Prapare ACM medium as described
- Prepare ACM agar plates
Gold Precipitation on ACM Plates
- Culture D. acidovorans for 3 days on agar plates at 30°C
- Overlay agar plates with 0.2% HAuCl4 in 0.5 % soft agarose
Result
- Gold precipitation on ACM agar plate worked even better than described in paper
Gold Precipitation in D. acidovorans Supernatants
- Culture D. acidovorans for 3 days in ACM plates
- Centrifuge bacteria in liquid culture (10 min 6,000 rpm)
- Add 0.2% HAuCl4 to supernatant and medium control
Result
- Gold precipitation with D. acidovorans supernatant worked well
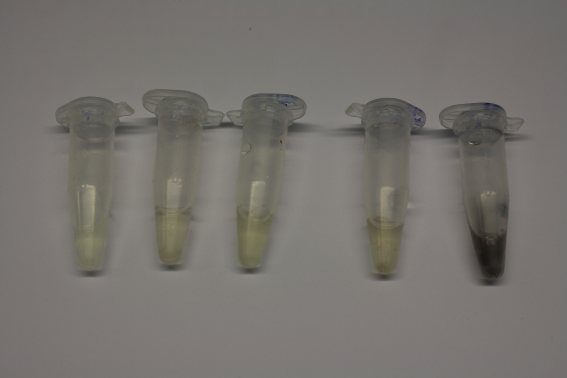
Gold Precipitation in D. acidovorans Supernatants at different Gold Chloride Concentrations
- Culture D. acidovorans for 3 days in ACM plates
- Centrifuge bacteria in liquid culture (10 min 6,000 rpm)
- Add HAuCl4 to supernatant and medium control
Result
- Gold precipitation with supernatant worked at very low concentration Gold
- Gold precipitation with unpurified culture worked as well
13-05 - 19-05-13
Gold Recovery from Electronic Waste
As test experiment, pins from an old CPU processor chip were dissolved.
Step 1: Pins were mechanically removed and collected in a flask.
Step 2: Flask was put onto heating plate and covered in concentrated HCl.
Step 3: HNO3 was carefully added to the pins and heated.
Step 4: After several minutes of moderate heating, pins dissolved completely. The solution turned green.
Step 5: The solution was carefully neutralized to pH ~6 in a step-wise manner using first 5 M, later 0.5 M NaOH.
Step 6: This led to precipitation of copper salts, indicated by the milky green coloring.
Step 7: In order to remove the precipitates, the solution was filtered using a buechner funnel and sterile filtrated (0.2 µm).
Step 8: The final yellow-green gold containing solution was collected in a volumetric flask.
Precipitation of Gold from Electronic Waste
Protocol
In order to evaluate optimial cultivation temperature of D. acidovorans, glycerol stock was streaked on ACM plates and incubated at RT, 30°C and 37°C ON. The next day, plates were covered with 200 µl 0.5% soft agar supplemented with 27 µl "dissolved electronic waste" solution.
Result
D. acidovorans showed intermediate, postponed purple-black coloring upon addition of "dissolved electronic waste" soft agar. Strongest coloring was observed on plates incubated at room temperature and 30°C. Delftibactin expression seems to be significantly decreased in D. acidovorans cultivated at 37°C. Reduced coloring further indicates lower gold ion concentration in the solution recovered compared to th ecommercially availible gold chloride solution [100 g/l]
We could therefore prove capability of D. acidovorans to specifically recover solid gold from dissolved electronic waste.
05-08 - 11-08-13
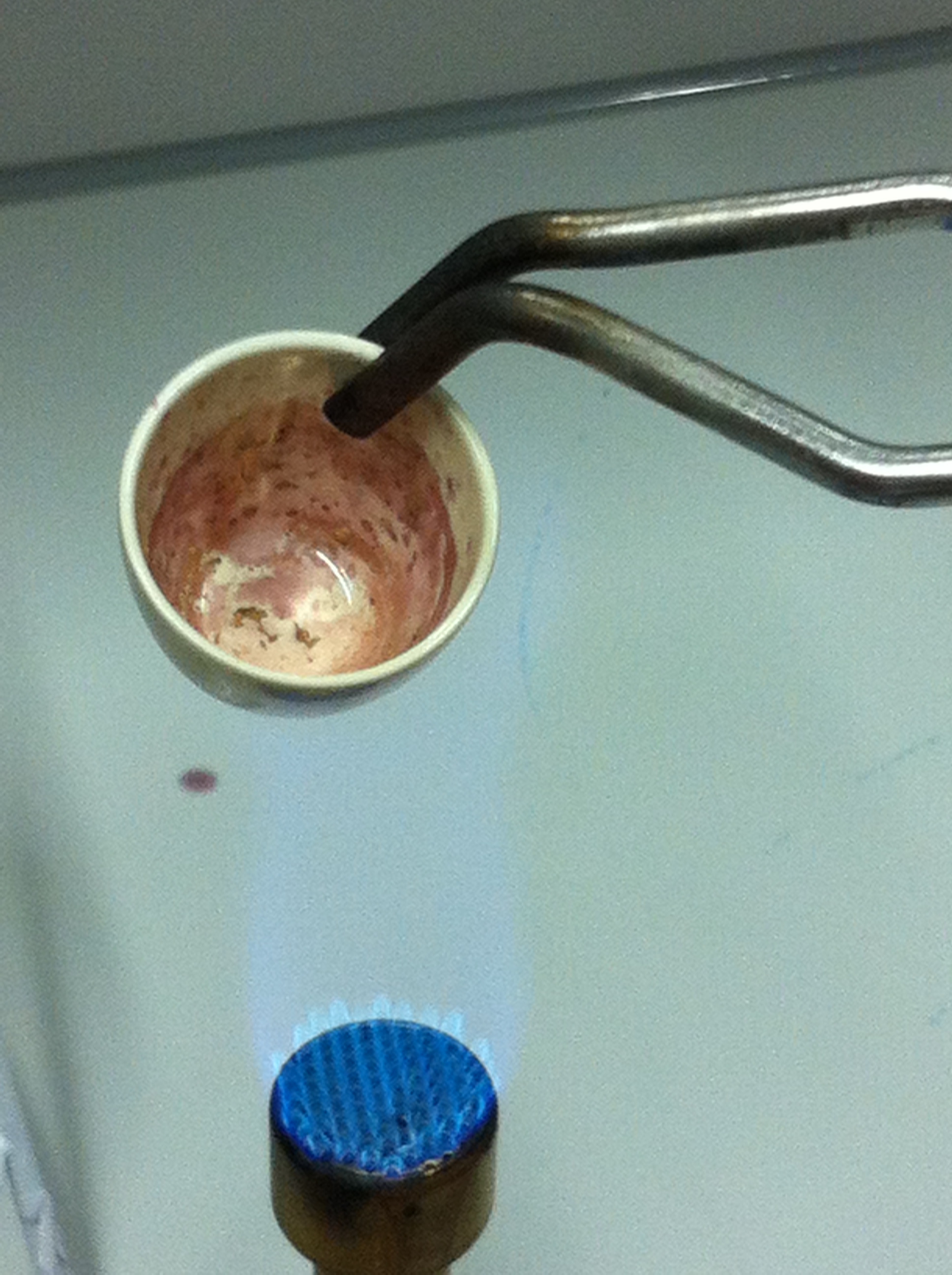
Melting Gold Nanoparticles to Solid Gold
Step 1: Cultivation of D. acidovorans
200 ml ACM medium were inocculated with recently obtained D. acidovorans SPH-1 from glycerol stock and incubated at 30°C for 3 days.
Step 2: Precipitation of Gold Nanoparticles
- D. acidovorans culture in ACM was divided in 50 ml aliquots and centrifuges at 3,750 rpm for 30 min.
- Supernatant was transferred to fresh tube.
- 300 µl gold chloride stock solution [µg/ml] was added to D. acidovorans supernatant, suspention mixed and incubated at RT for 20 min.
Step 3: Aggregation of gold nanoparticles.
Step 4: Melting gold nanoparticles to solid gold.
Alternatively, we also performed the protocol described above in a 2 ml tube.
02-09 - 08-09-13
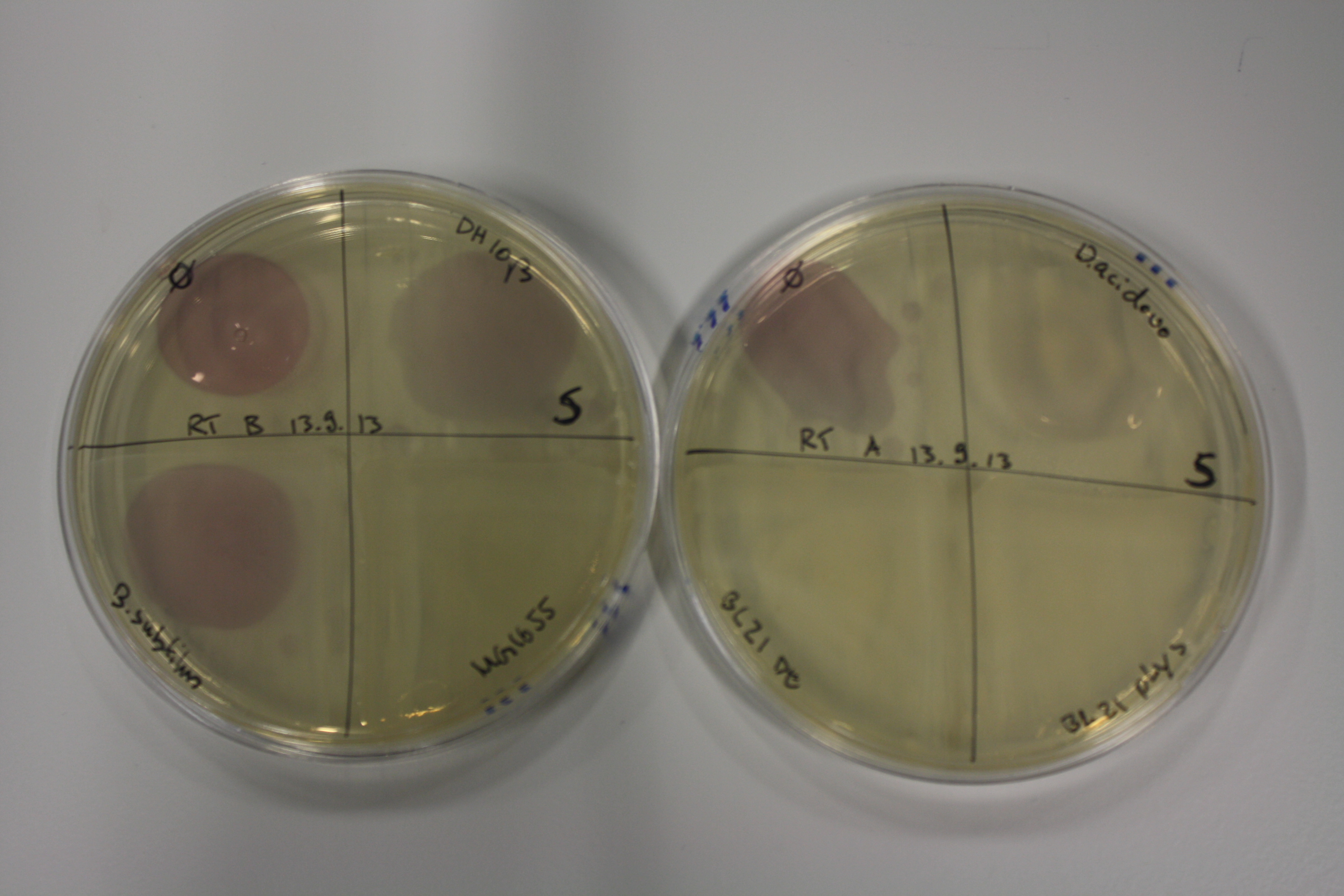
Optimizing Growth Conditions of D.acidovorans and Evaluation of Endogenous Background Gold Precipitation
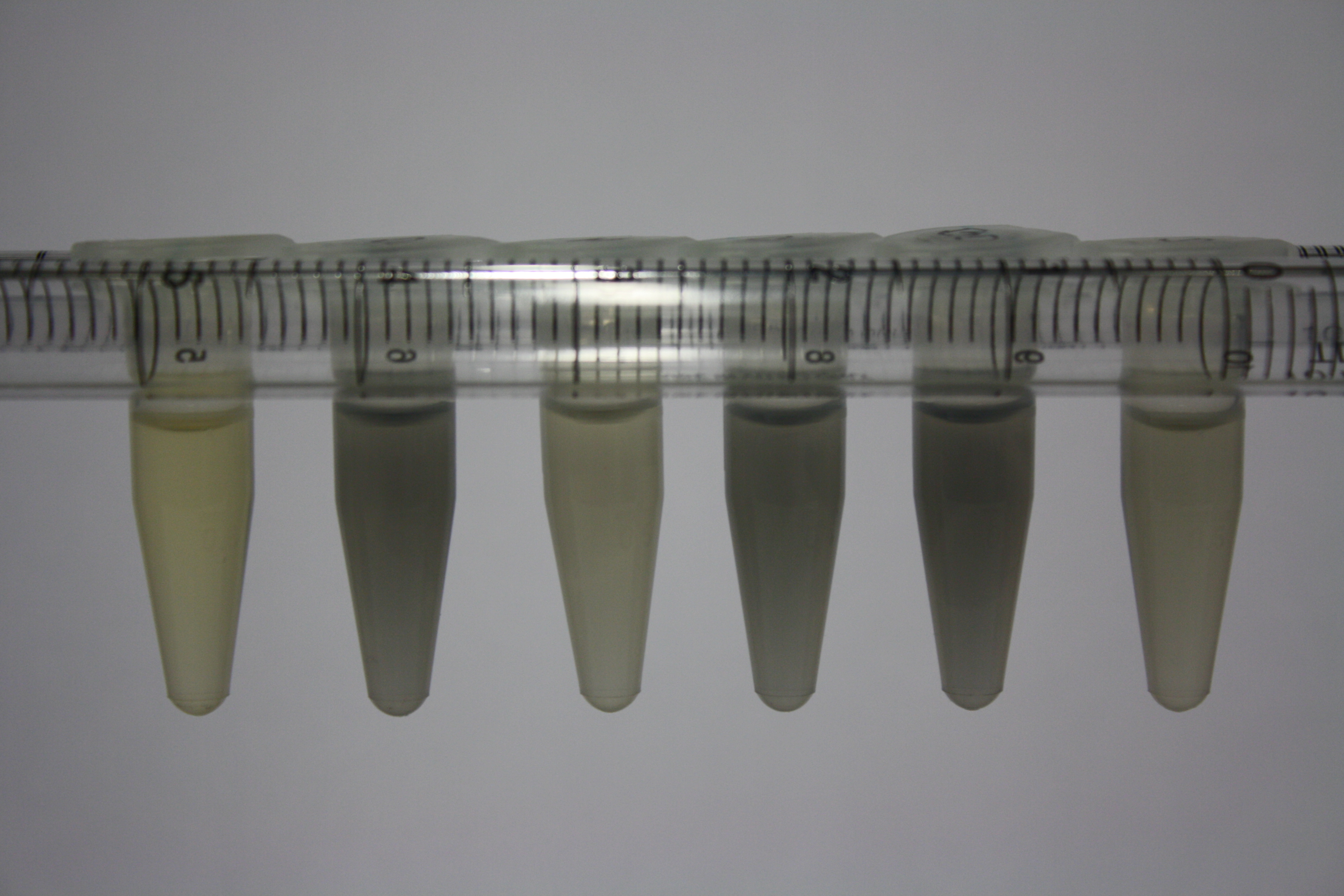
In order to optimize plates for cultivation of D.acidovorans as well as to evaluate of endogenous capability of possible target strains, capability of D.acidovorans and E. coli DH10ß and BL21 DE3 grown on LB and ACM plates was tested.
On Agar Plate following ON Incubation at 30°C
- On agar plates, D.acidovorans, E.coli DH10ß, and Bl21 were plated from liquid culture and grown ON at 30 °C.
- The following day, 1.6 ml soft agar (0.5%) was mixed with goldchloride [100 g/l] as follows:
| Final goldchloride concentration [mM] | Volume goldchloride stock [µl] |
|---|---|
| 5 | 27 |
| 10 | 54.4 |
| 20 | 108.8 |
| Agar plate | Goldchloride [mM] | H2O | D.acidovorans | DH10ß | BL21 DE3 |
|---|---|---|---|---|---|
| ACM | 0 | no reaction | no reaction | no reaction | no reaction |
| ACM | 5 | postponed weak coloring ON | weak coloring after 2 min | weak coloring after 2 min | weak coloring after 2 min |
| ACM | 10 | postponed dark coloring ON | strong coloring after 2 min | strong coloring after 2 min | strong coloring after 2 min |
| ACM | 20 | postponed dark coloring ON | strong coloring after 2 min | strong coloring after 2 min | strong coloring after 2 min |
| LB | 0 | no reaction | no reaction | no reaction | no reaction |
| LB | 5 | no reaction | no reaction | no reaction | no reaction |
| LB | 10 | no reaction | no reaction | no reaction | no reaction |
| LB | 20 | no reaction | no reaction | no reaction | no reaction |
Result
All bacteria showed strong and immediate purple-black coloring indicating gold precipitation depending on gold chloride concentration. Regarding the precipitation of gold, there does not seem to be a significant advantage of D.acidivorans. In contrast to the paper from Johnston et al., we only grew them ON at 30°C rather than for 3 days. We will repeat the experiment using 3 day incubation time and also evaluate gold precipitation on additional media as well as by additional bacteria.
09-09 - 15-09-13
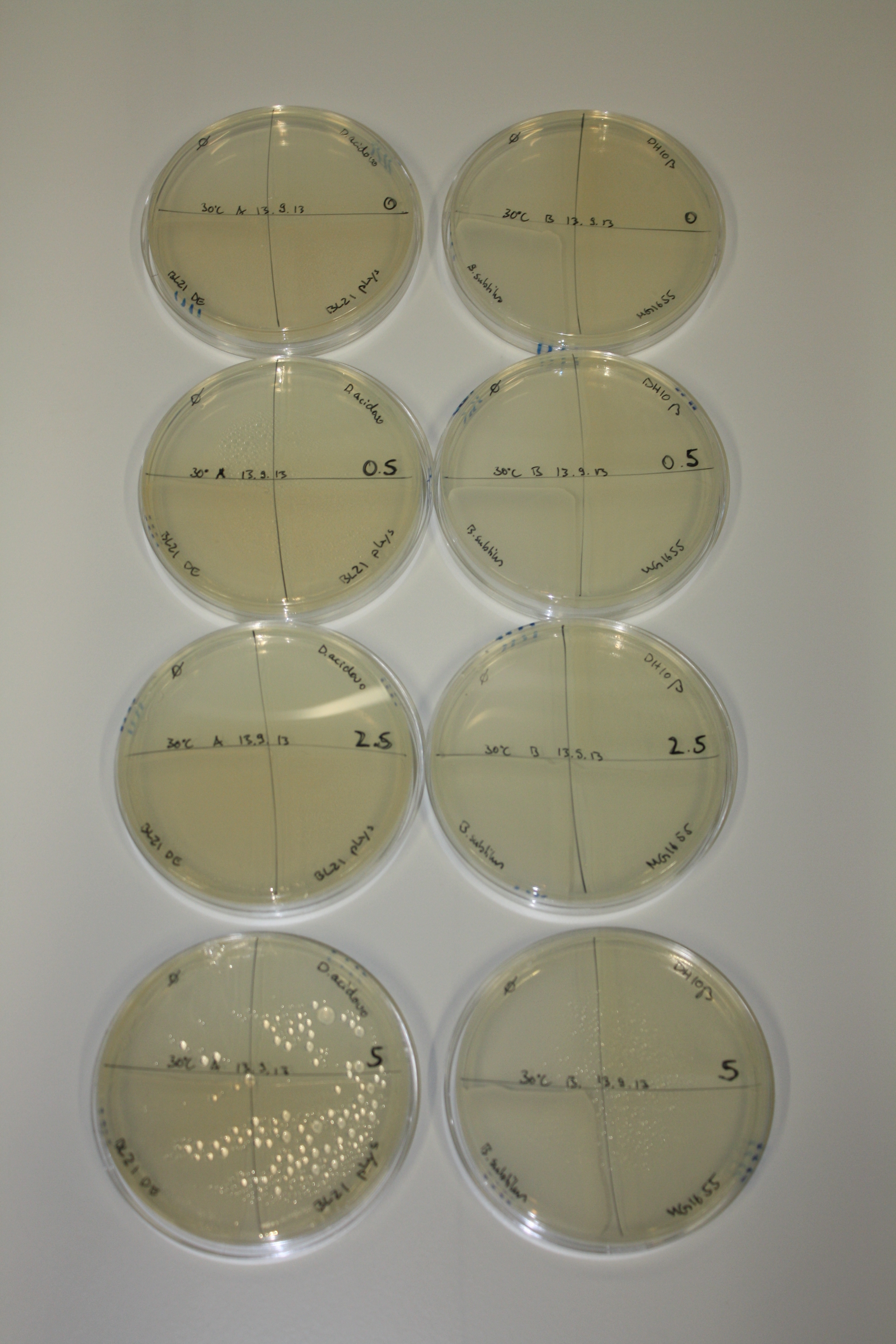
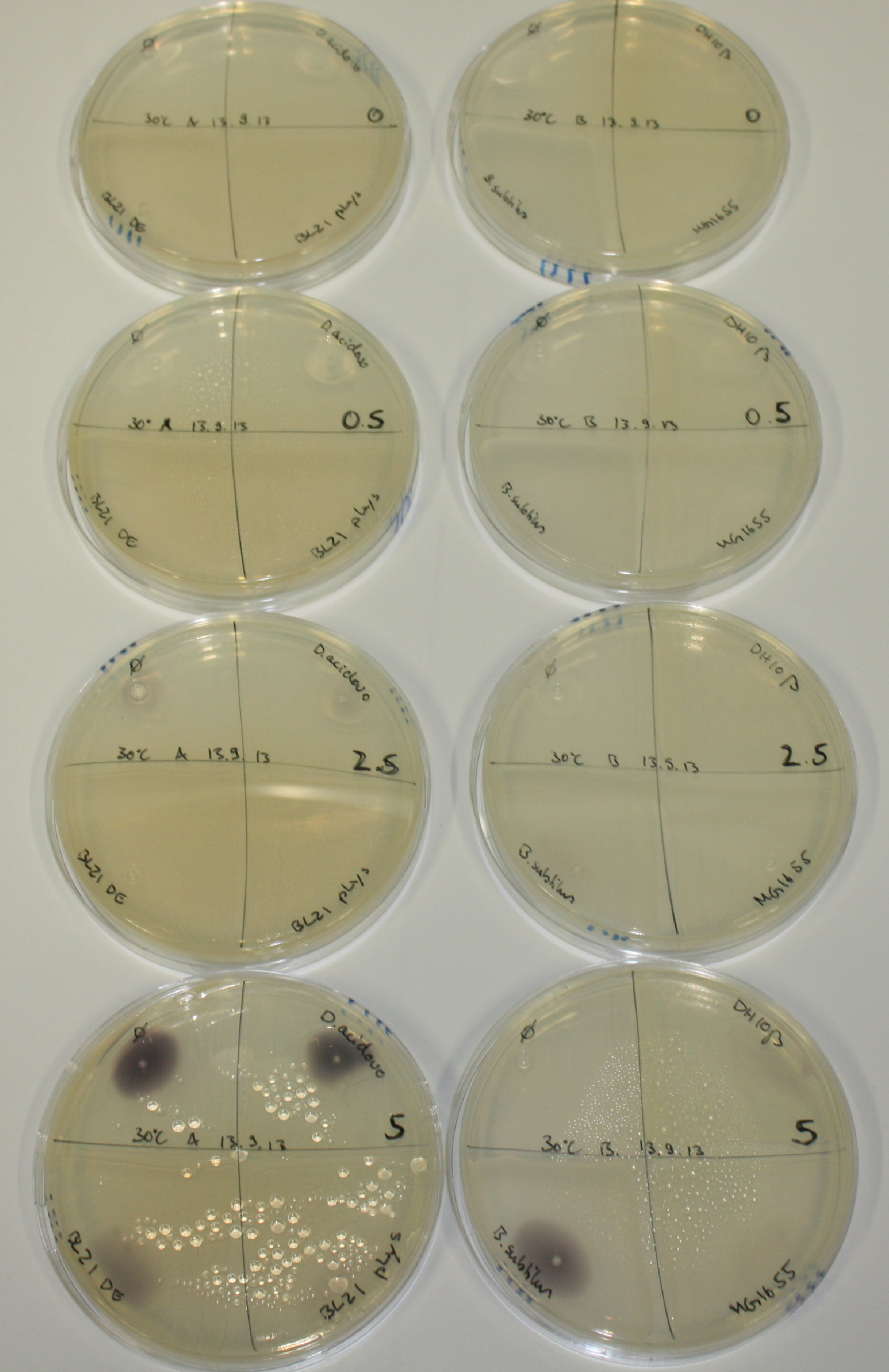
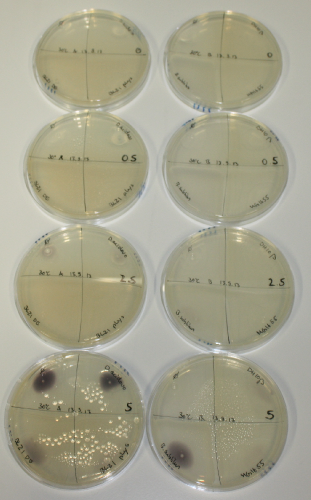
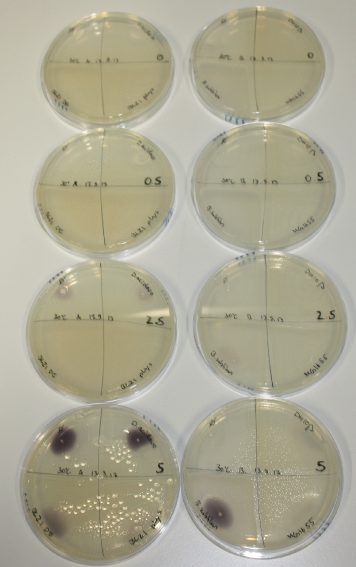
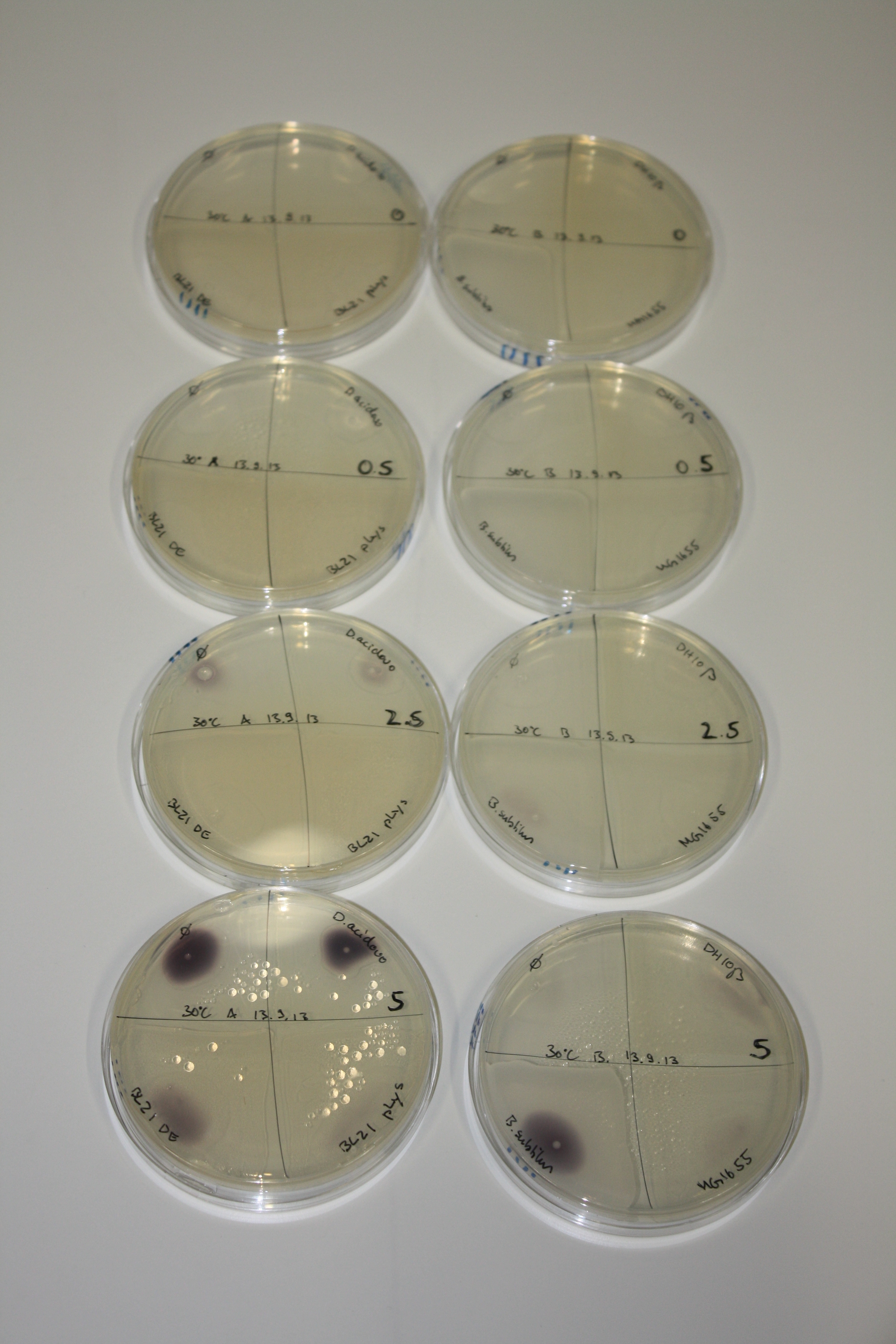
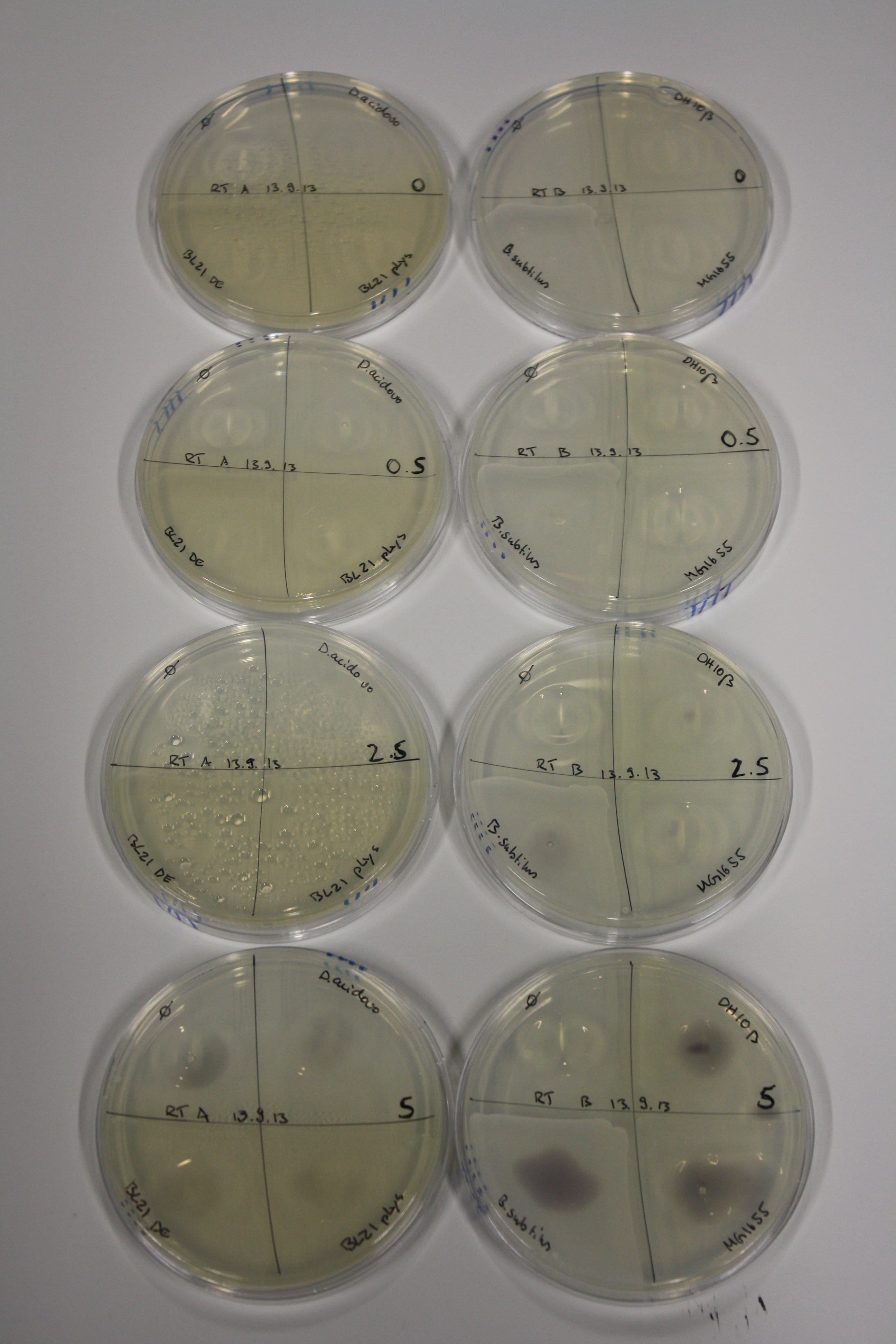
Optimizing Growth Conditions of D.acidovorans and Evaluation of Endogenous Background Gold Precipitation
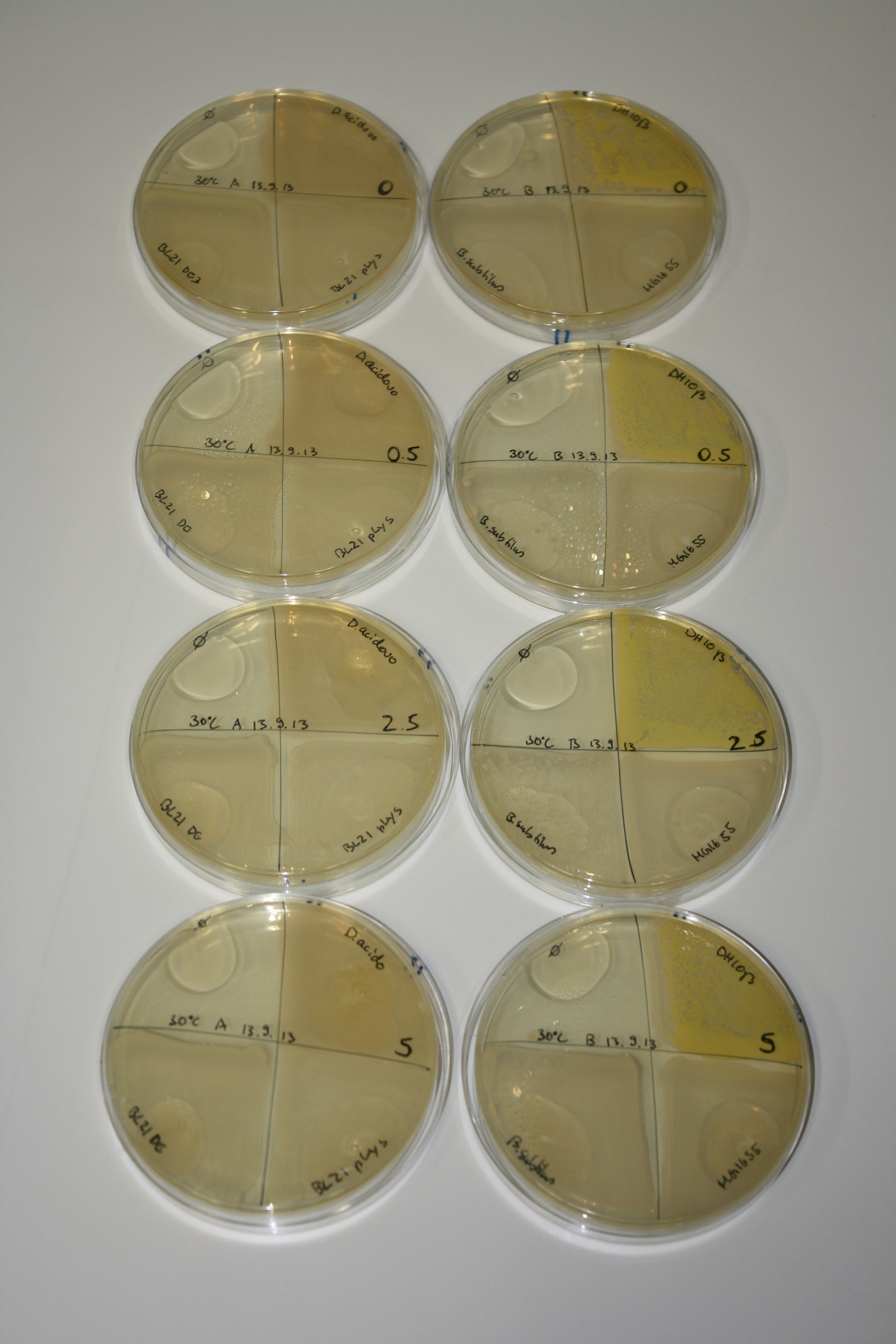
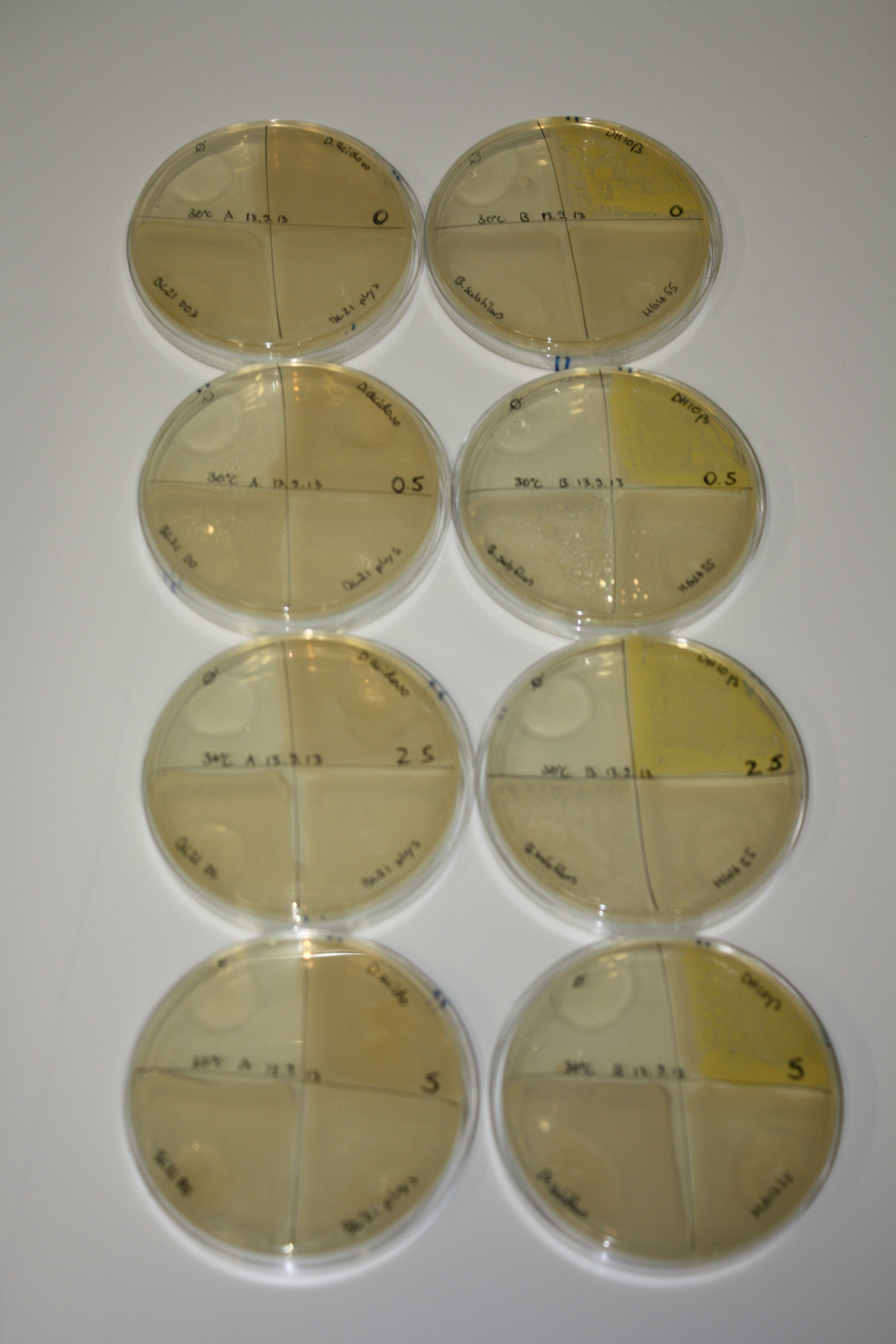
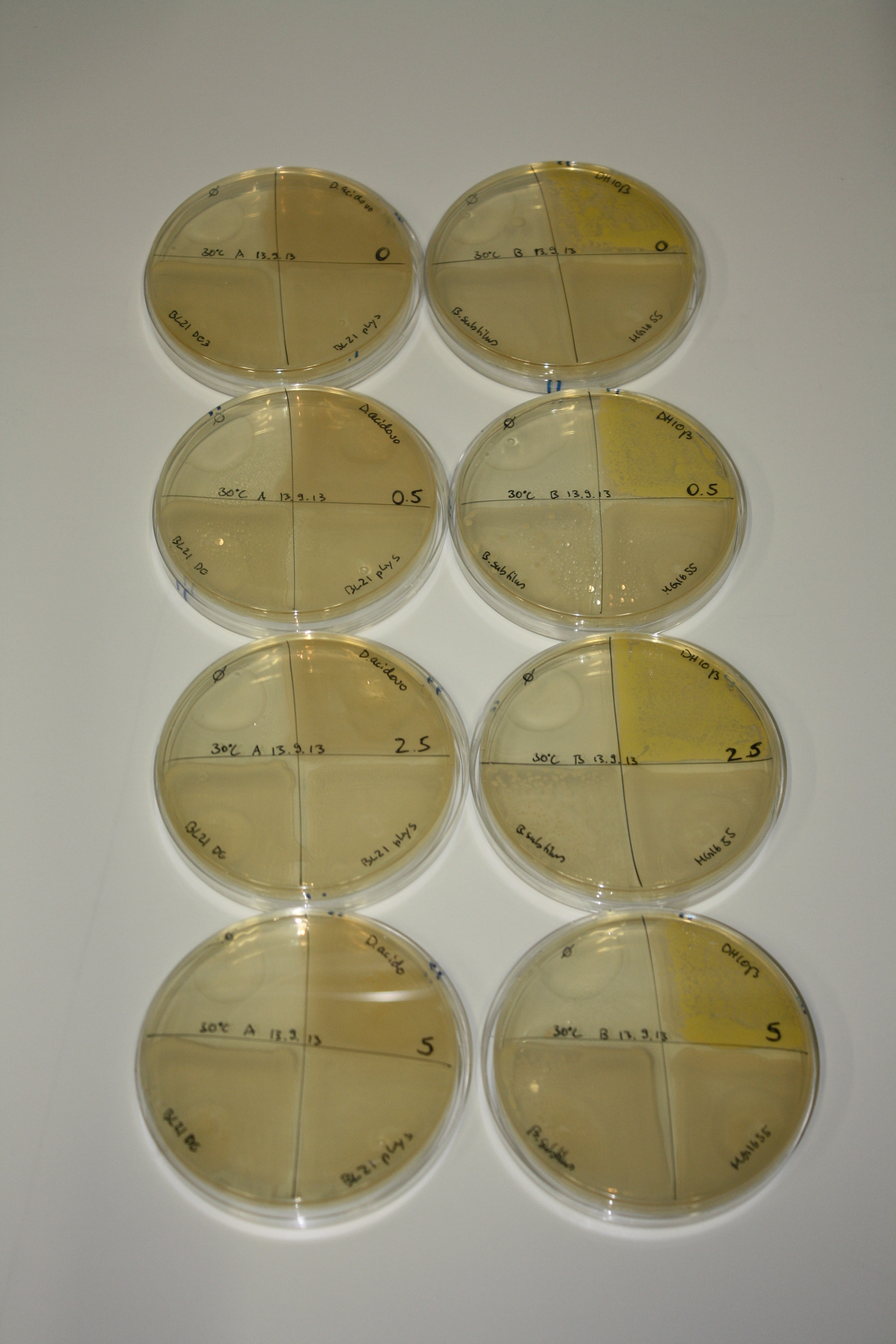
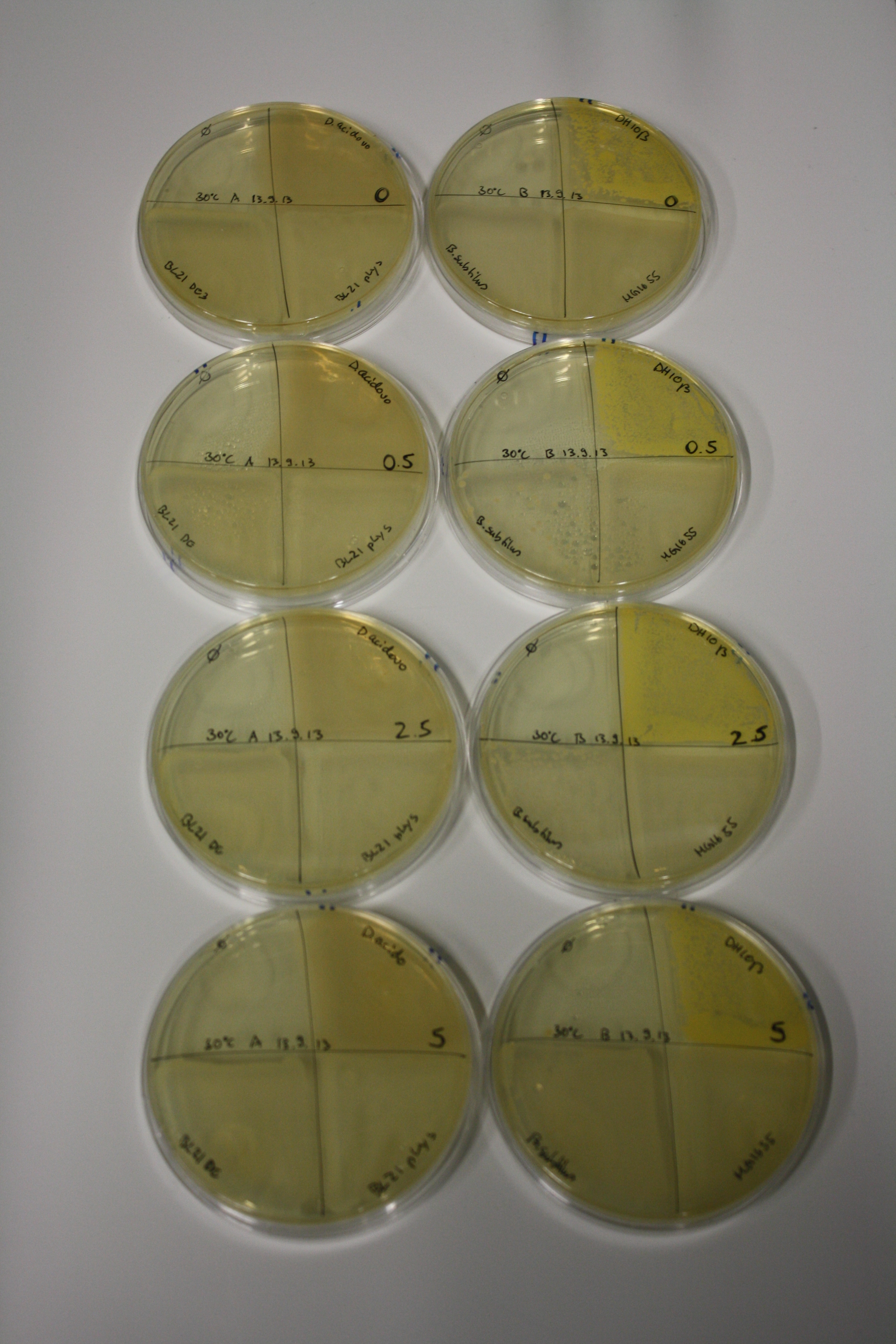
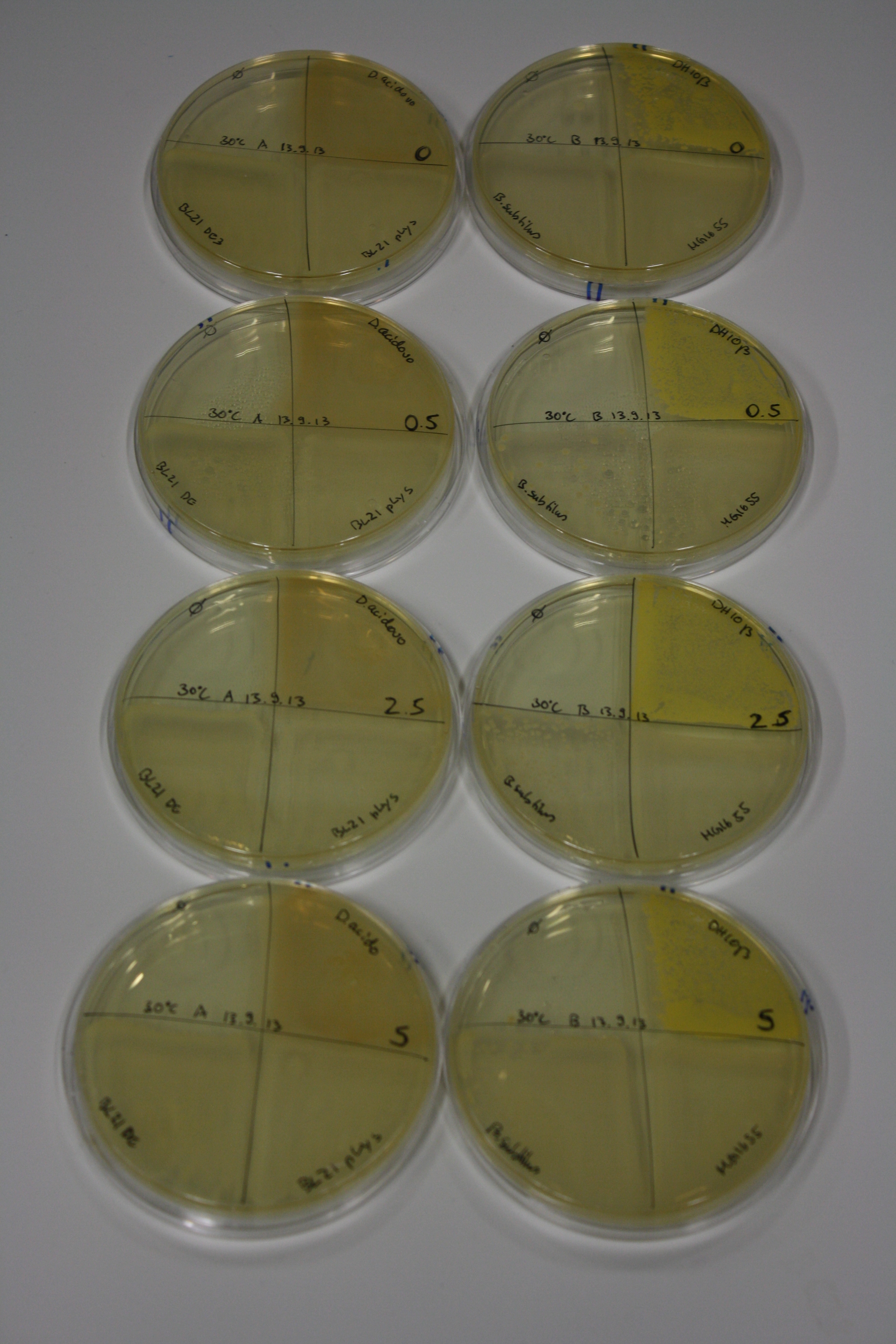
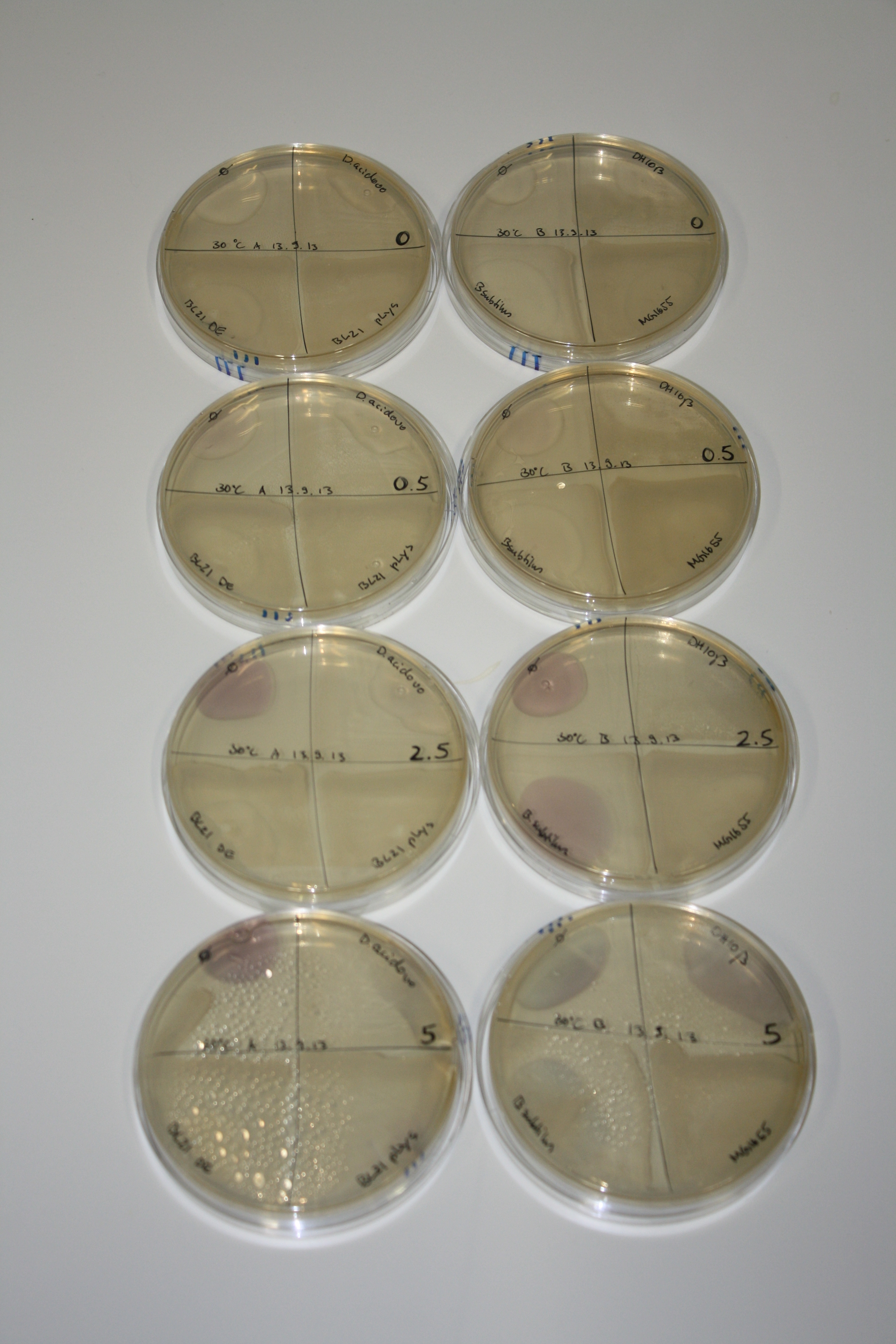
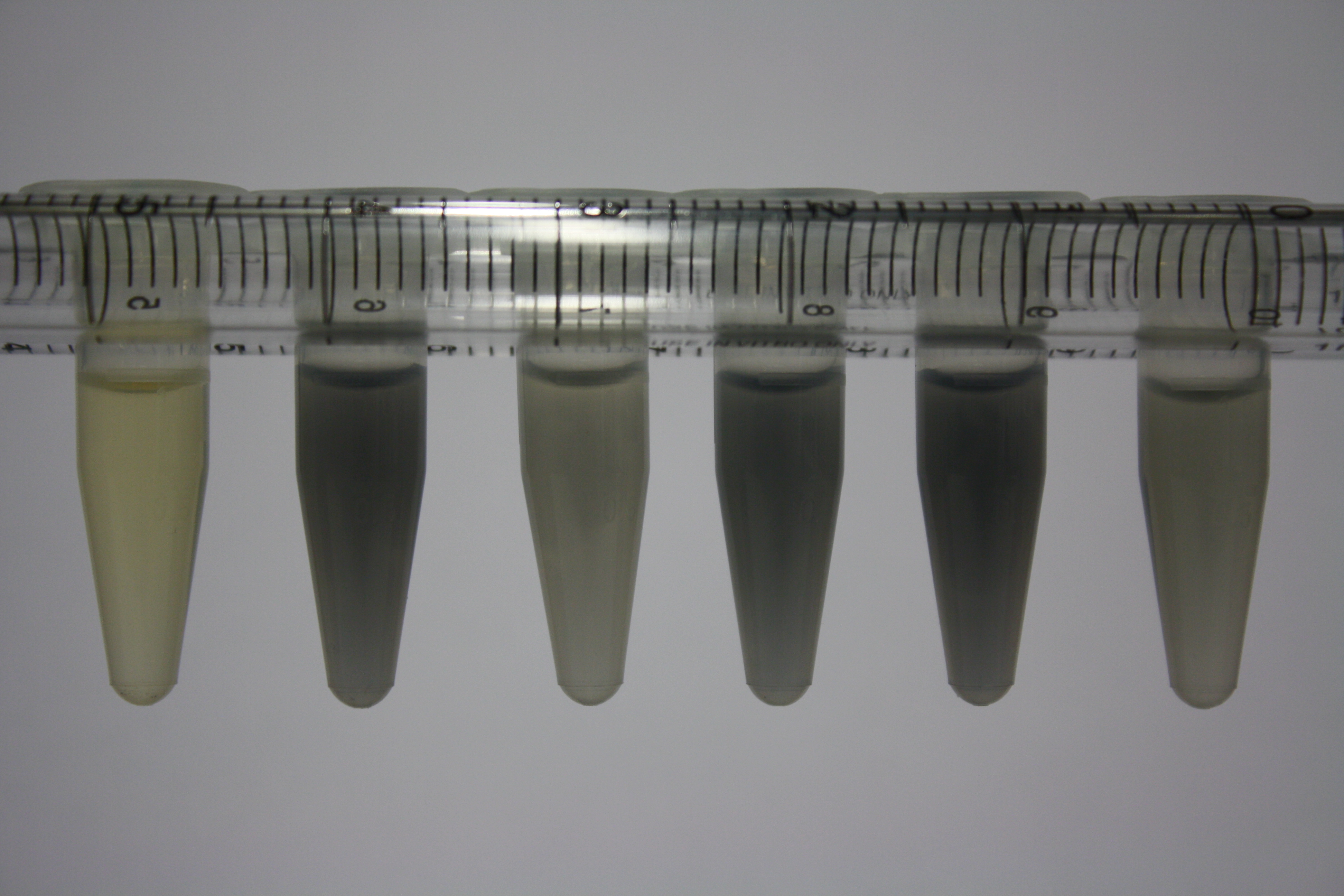
In order to optimize plates, growth time and incubation temperature of D.acidovorans as well as to evaluate of endogenous capability of possible target strains, various growth conditions and bacteria were tested.
On Agar Plate following 3 Day Incubation at 30°C
- Media was chelex treated, agar added and autoclaved, before plates were poured.
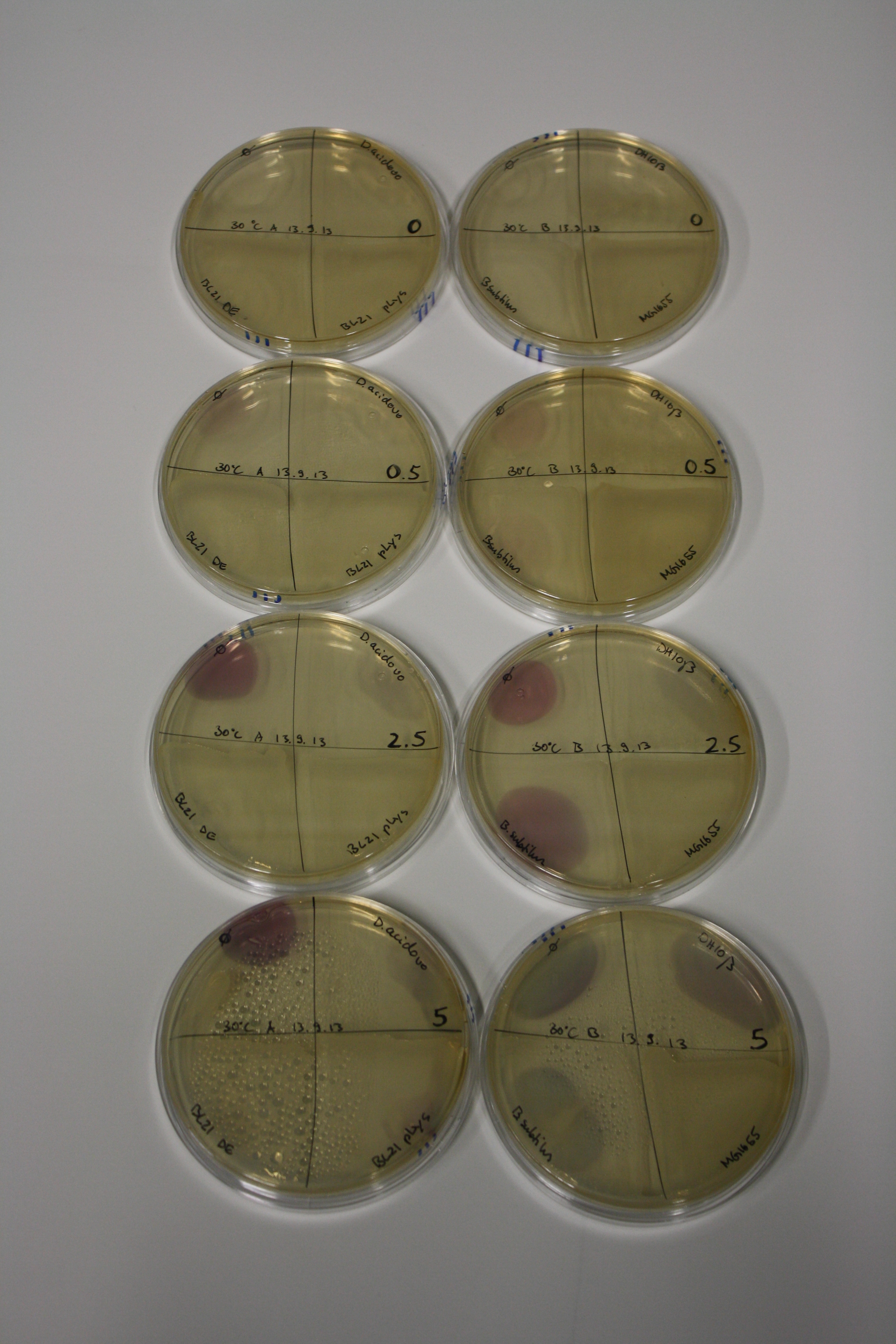
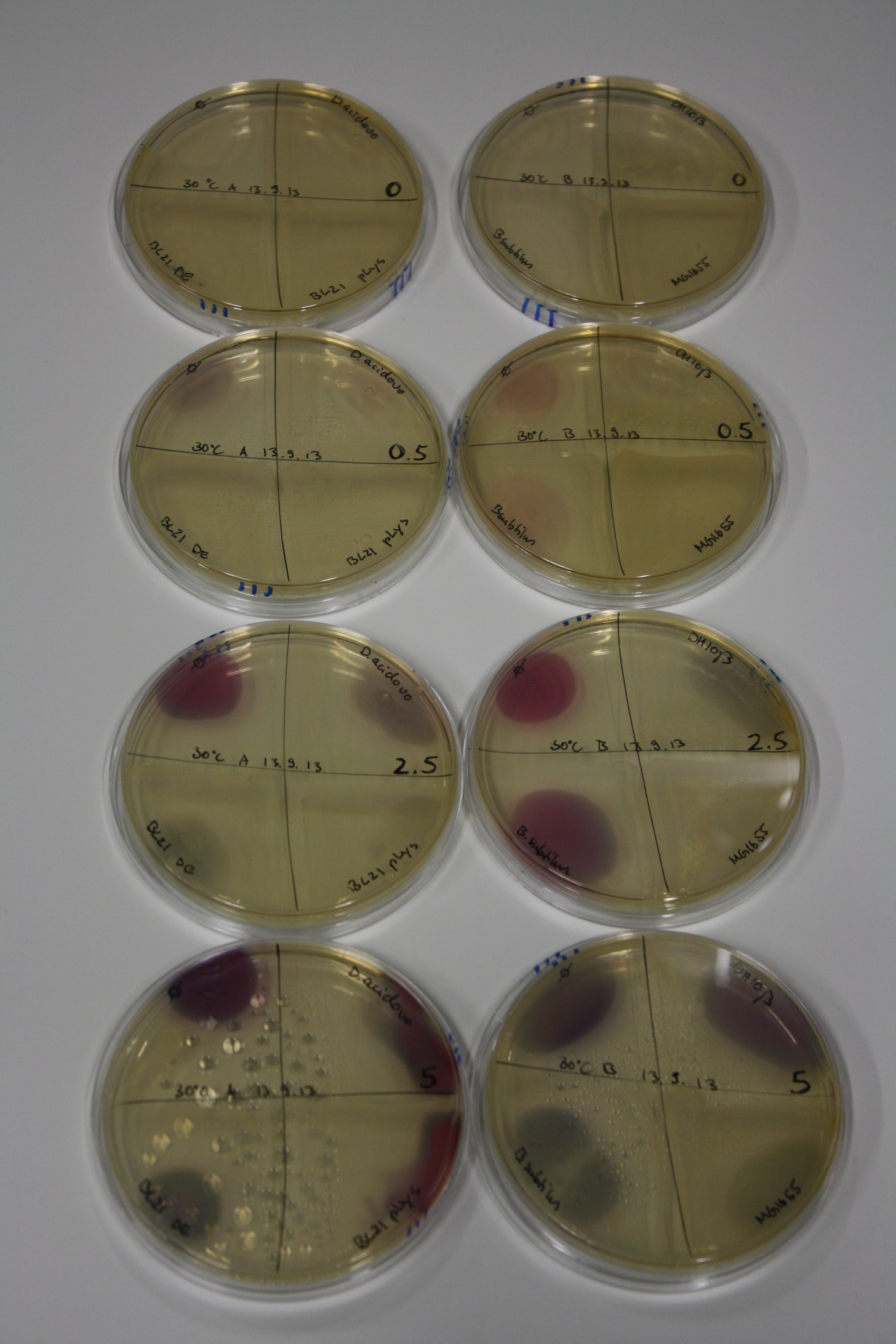
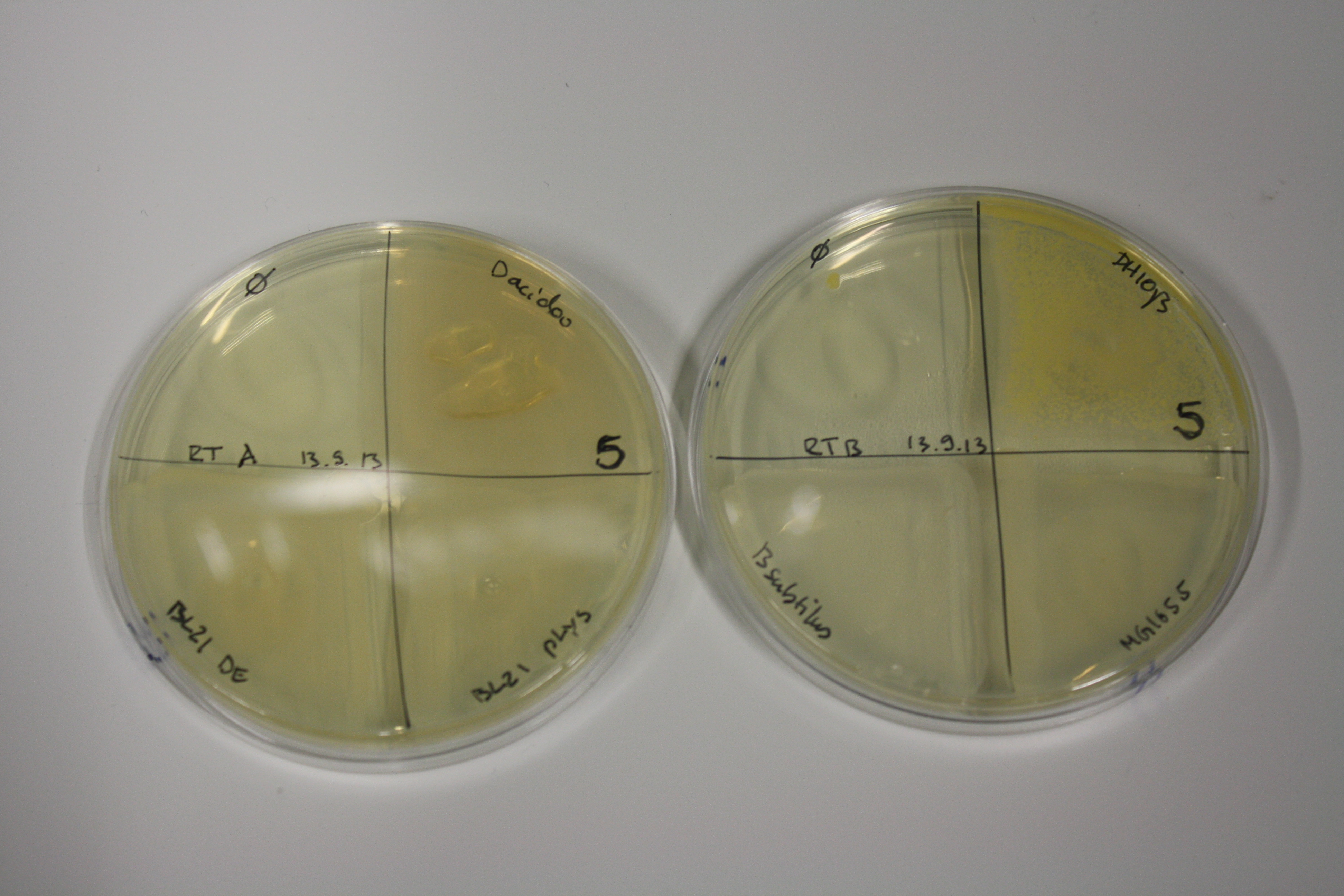
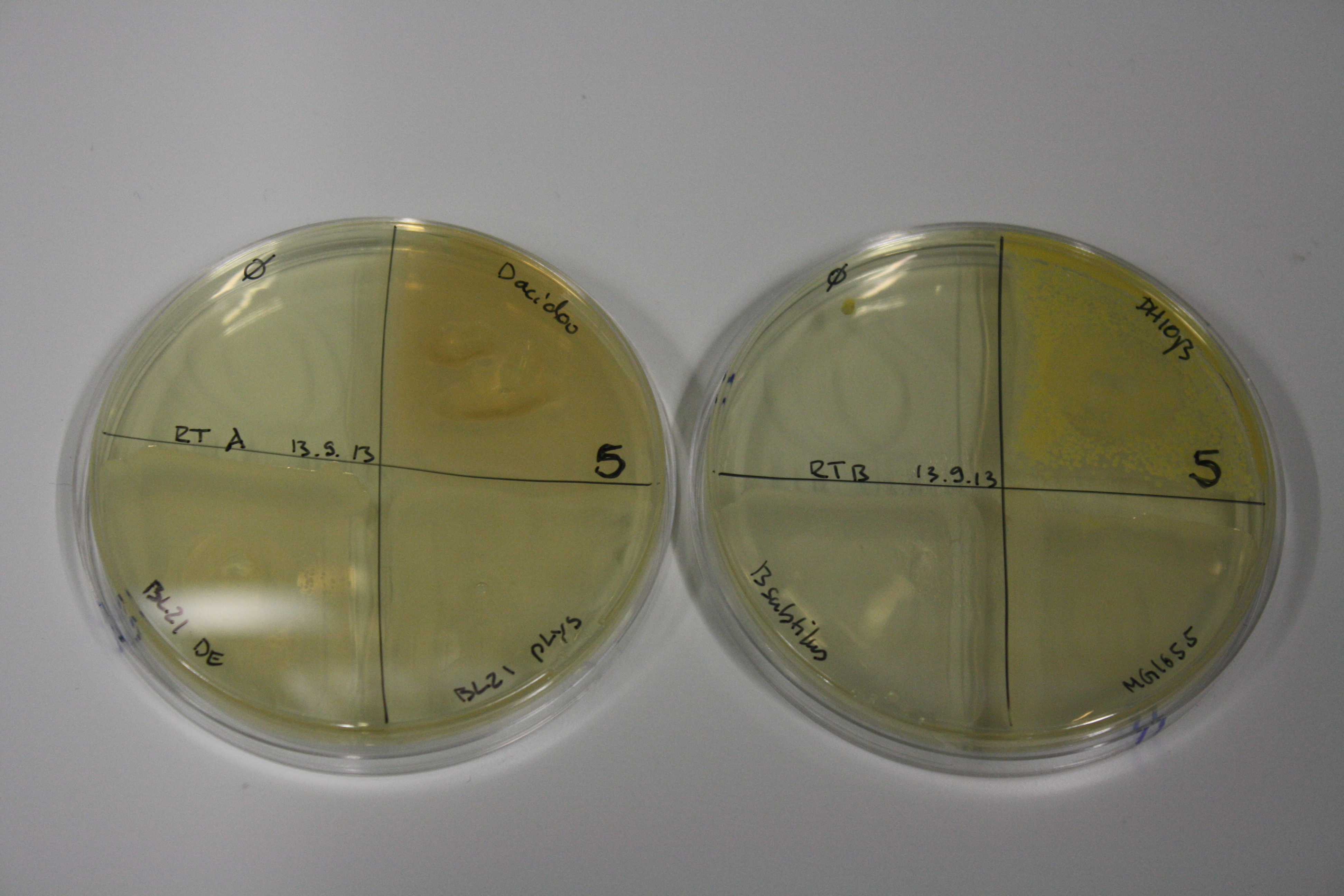
- On agar plates, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 and Bacillus subtilus were plated from liquid culture and grown for three days at 30 °C.
- 14.4 ml soft agar (0.5%) was mixed with gold chloride [100 g/l] as follows and 200 µl per quarter plate added:
| Final gold chloride concentration [mM] | Volume goldchloride stock [µl] |
|---|---|
| 0 | 0 |
| 0.5 | 2.7 |
| 2.5 | 13.5 |
| 5 | 27 |
Result
| Agar plate | Goldchloride [mM] | H2O | D.acidovorans | BL21 DE3 | BL21 pLys | H2O | DH10ß | B.circulans | MG1655 |
|---|---|---|---|---|---|---|---|---|---|
| ACM | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| ACM | 0.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
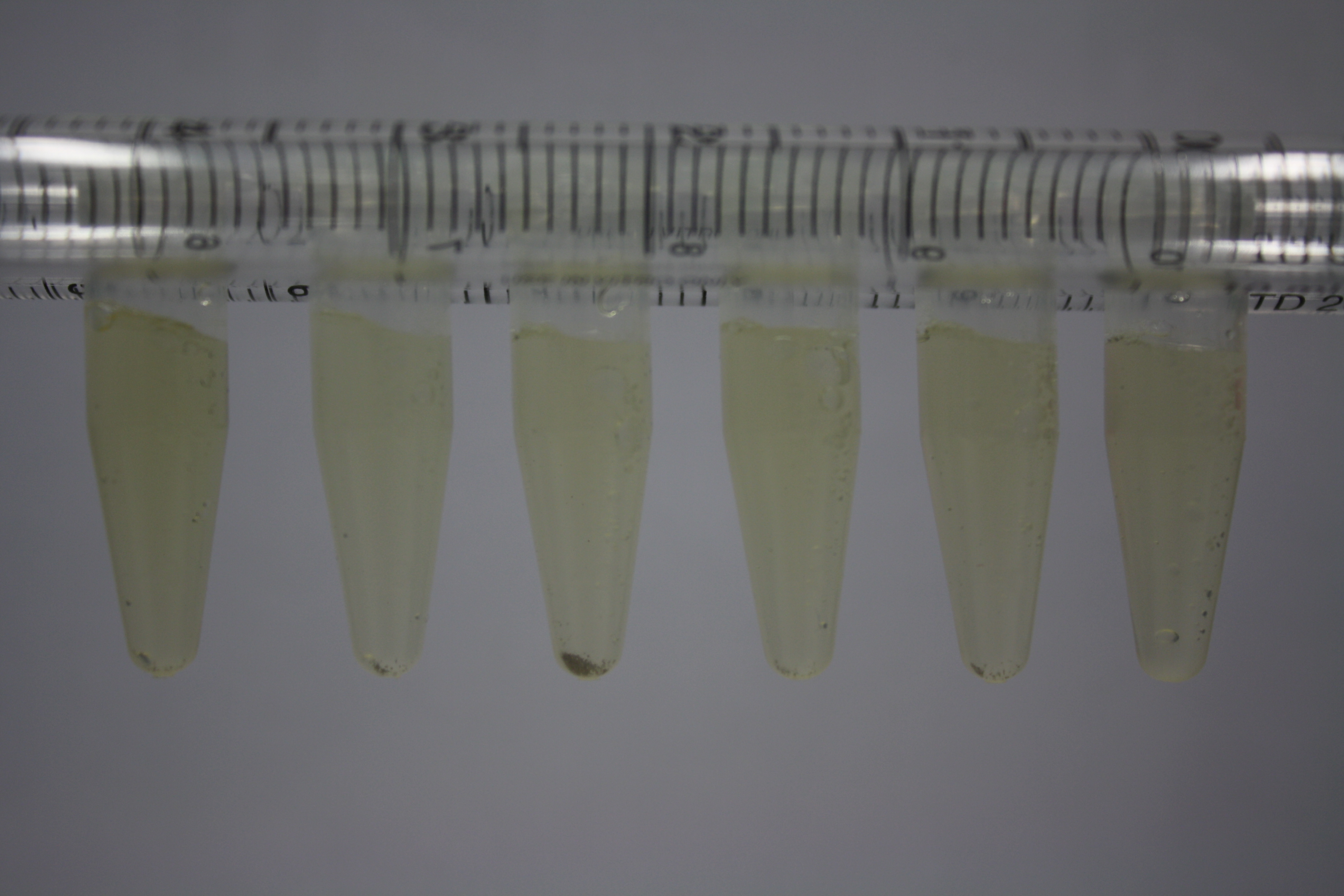
| ACM | 2.5 | minor coloring | minor coloring | no reaction | no reaction | no reaction | no reaction | minor coloring | no reaction |
| ACM | 5 | strong coloring | strong coloring | intermediate coloring | minor coloring | minor coloring | minor coloring | minor coloring | minor coloring |
| LB | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| LB | 0.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| LB | 2.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| LB | 5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 0.5 | minor coloring | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 2.5 | strong coloring | minor coloring | no reaction | no reaction | strong coloring | minor coloring | strong coloring | no reaction |
| M9 | 5 | strong coloring | minor coloring | no reaction | no reaction | minor coloring | minor coloring | minor coloring | no reaction |
Unfortunately, the streaked E.coli DH10ß were contaminated (obvious only on LB plates) and can threfore not be analyzed.
On ACM plates following 30°C incubation, D.acidovorans, B.circulans, E.coli BL21 DE3 as well as the negative control next to D.acidovorans, but not the one on plate B, showed immediate purple-black coloring indicating formation of gold nanoparticles at 5 mM gold chloride. At 2.5 mM, D.acidovorans and the neighbouring negative control as well as B.circulans showed some coloring. E.coli BL21 pLys and MG1655 only showed minor coloring at 5 mM. At lower concentrations, no gold precipitation was observed.
- => E.coli BL21 pLys and MG1655 are promissing candidates for expression since they show the least background activity.
- => Optimal gold chloride concentration lies between 2.5 and 5 mM.
- => Delftibactin can diffuse through agar.
On LB plates following 30°C incubation, none of the tested bacteria showed any reaction.
- => LB is not suitable for expression of NRPs.
- => What is missing in LB that does not allow metall precipitating activity?
On M9 plates following 30°C incubation (which were mistakenly autoclaved after addition of glucose), D.acidovorans hardly grew. At 5 mM and 2.5 mM gold chloride, there is heavy background activity on the negative controls. Additionally, B.circulans shows strong - D.acidovorans minor formation of nanoparticles.
- => M9 is not suitable for gold precipitation experiments.
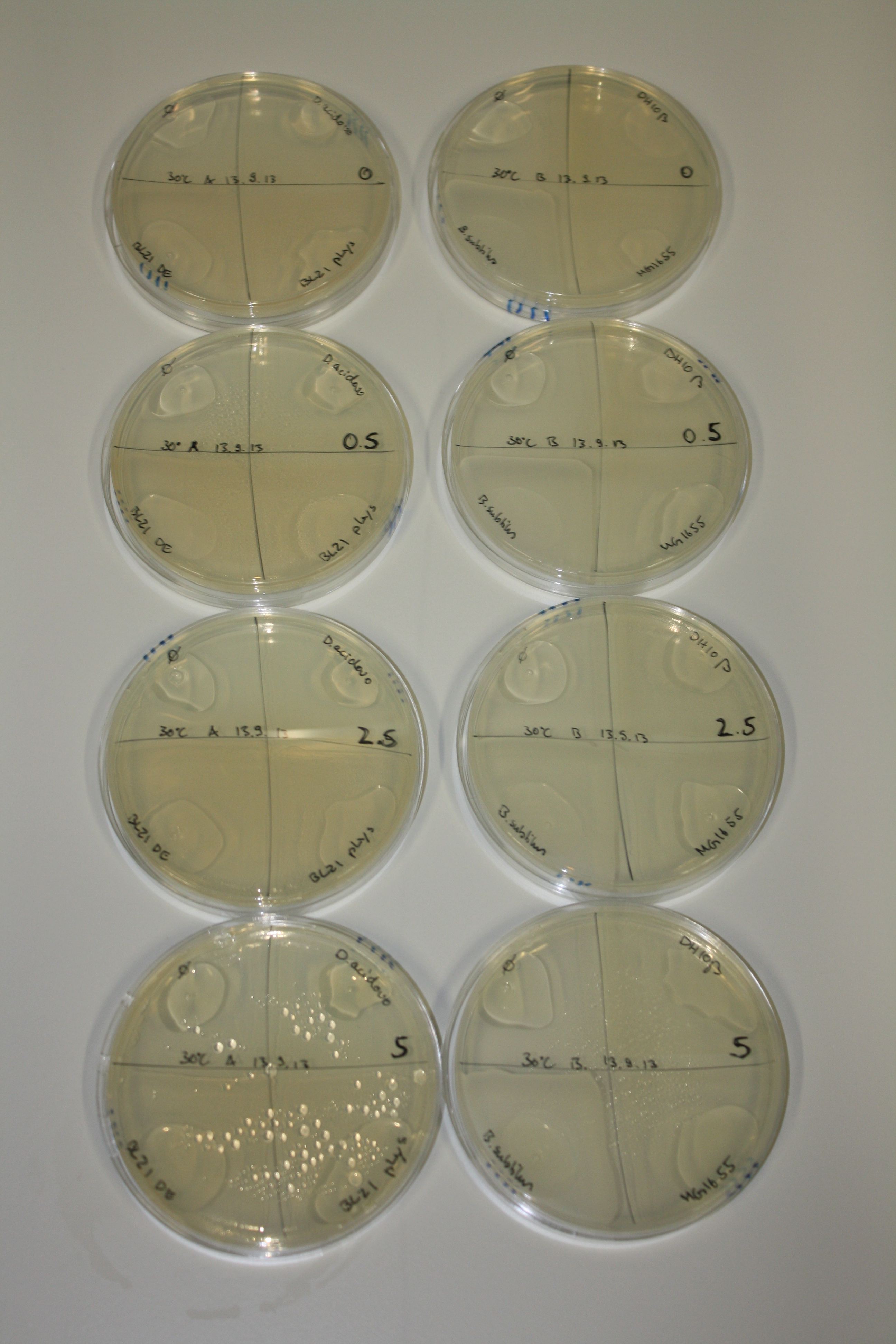
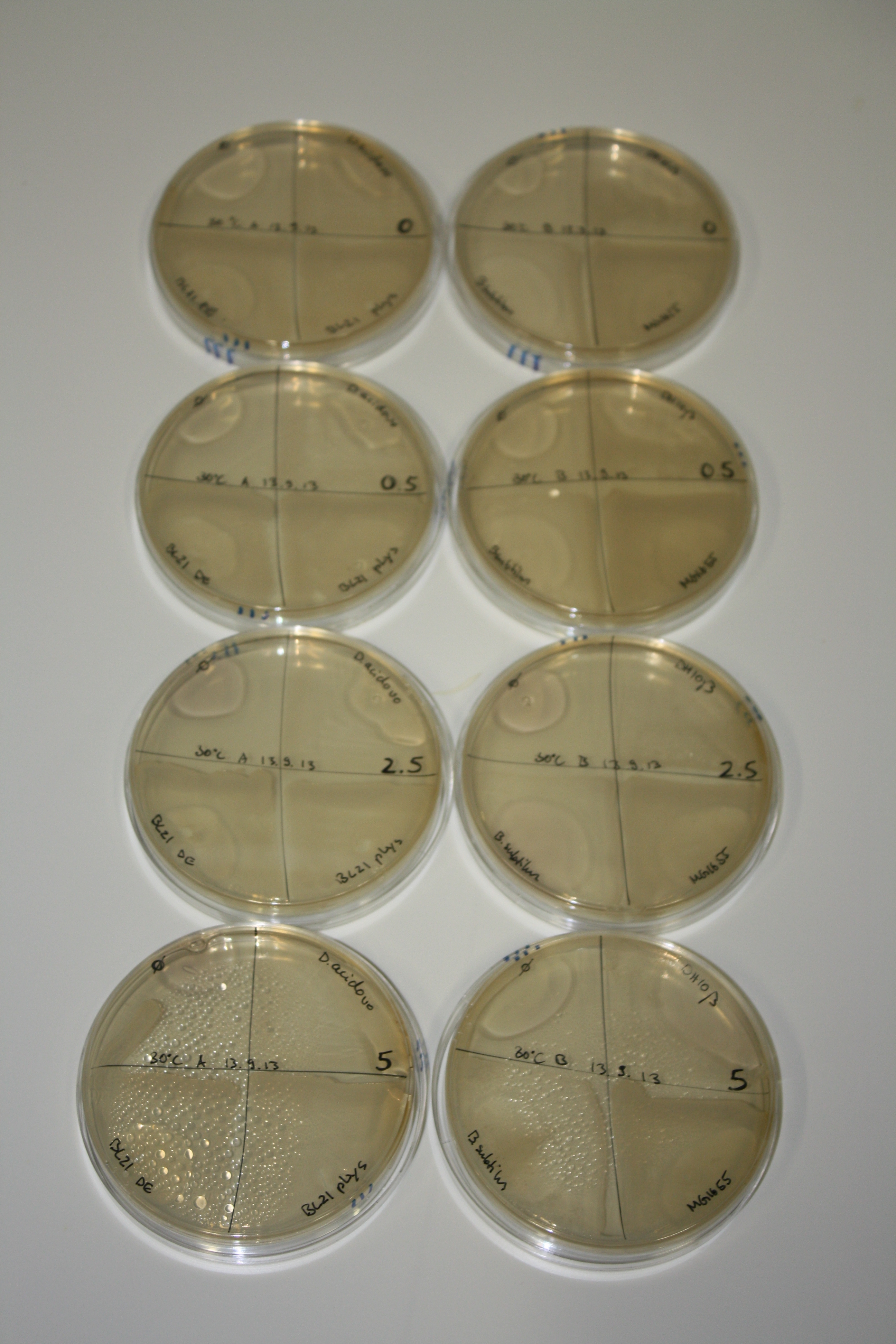
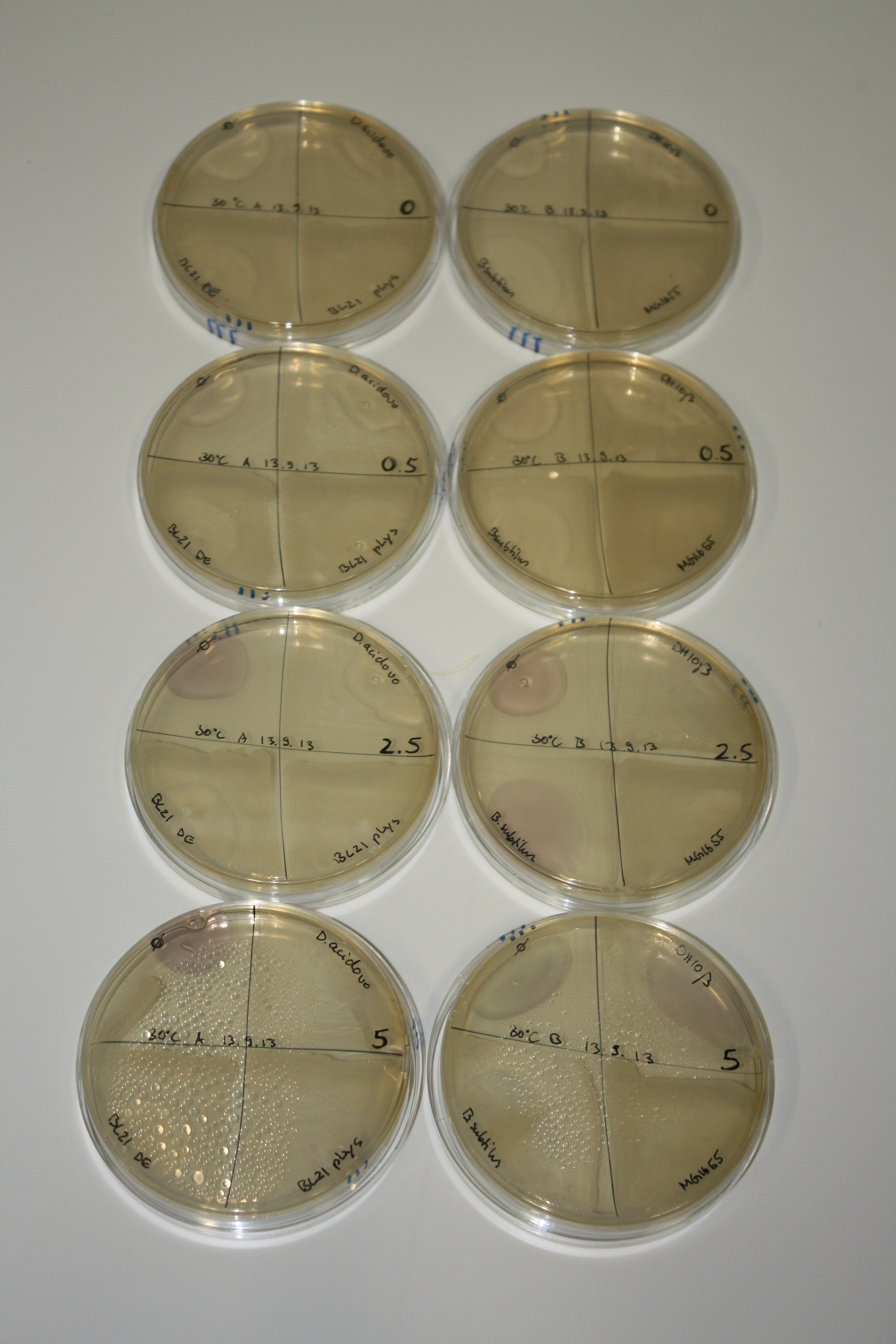
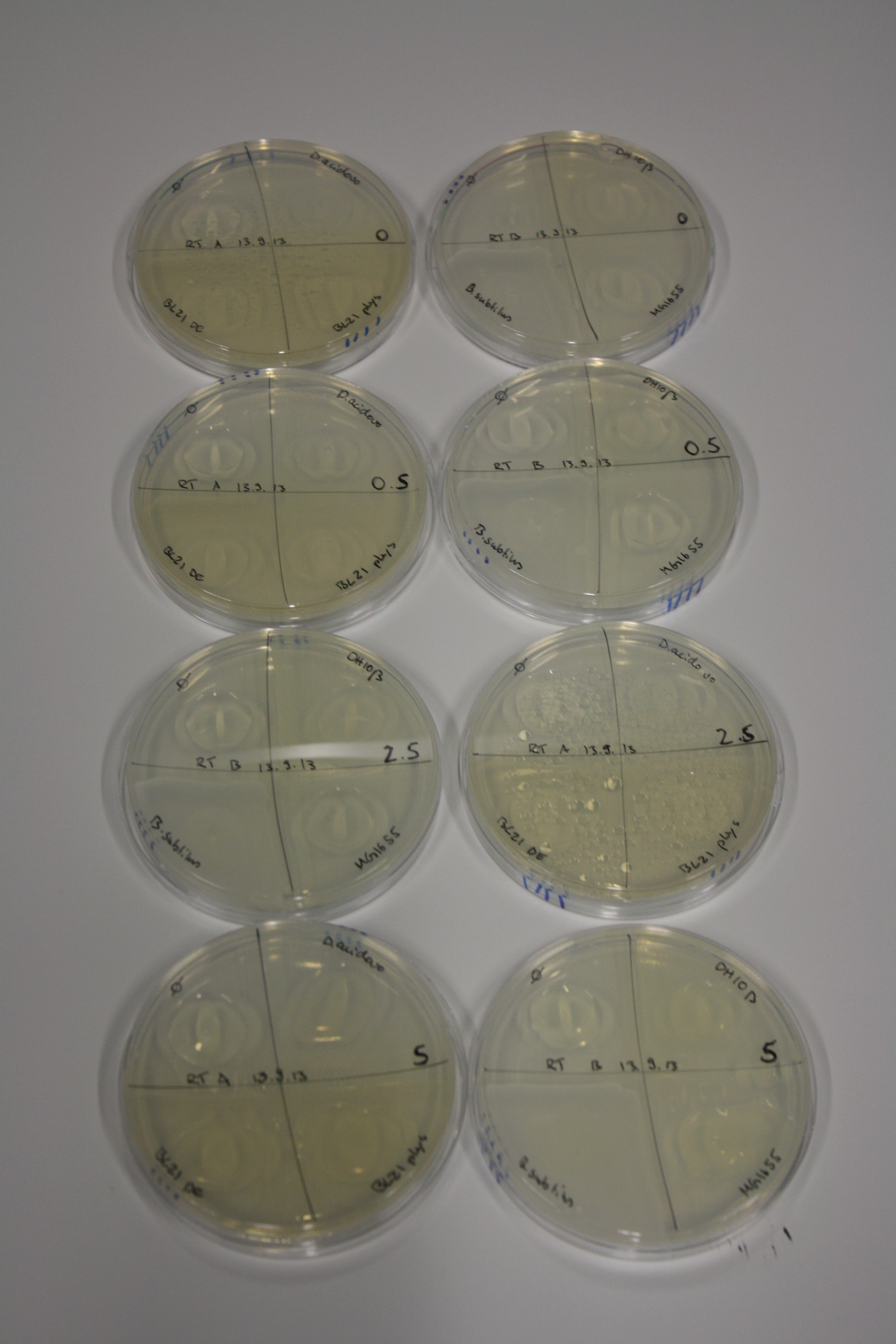
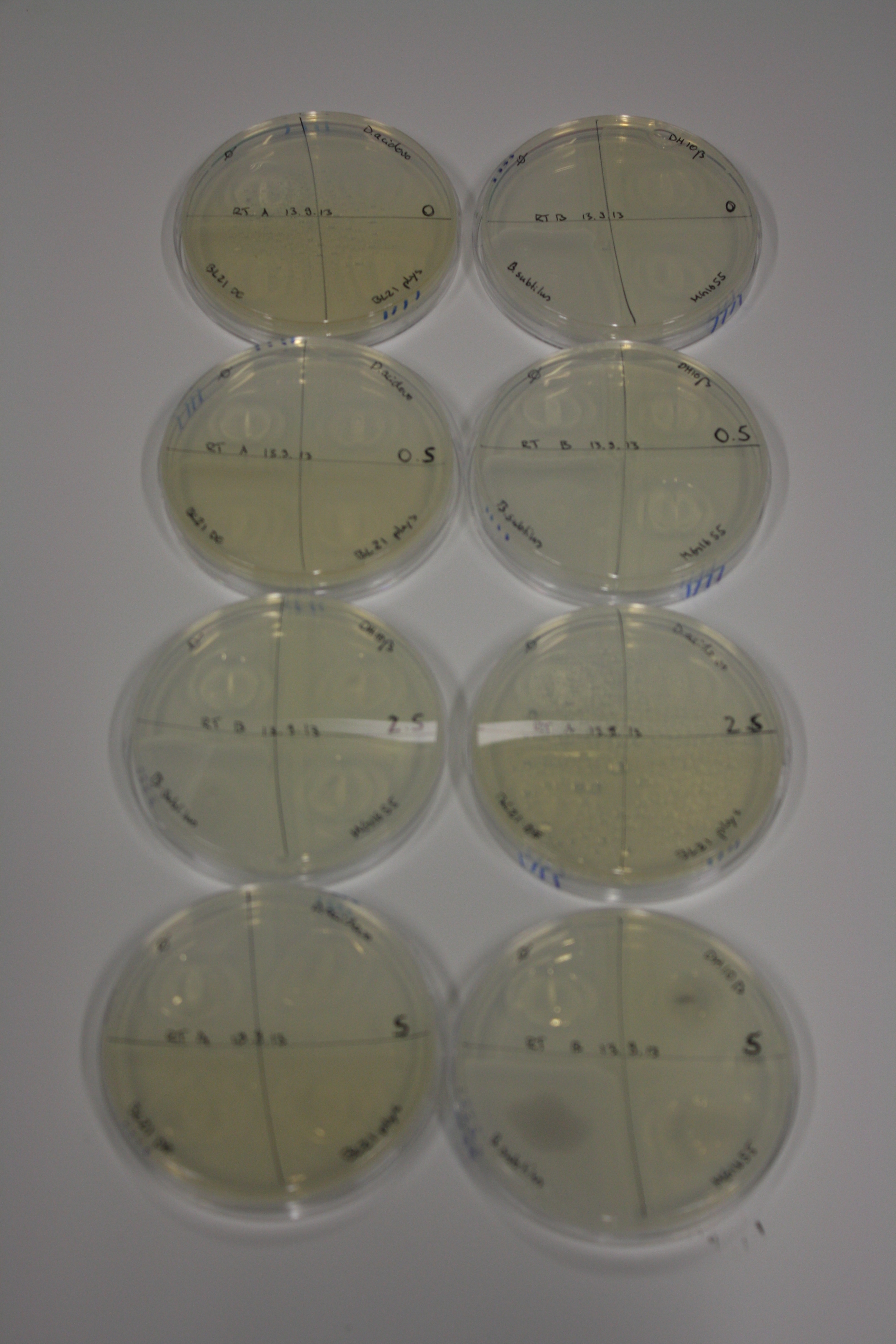
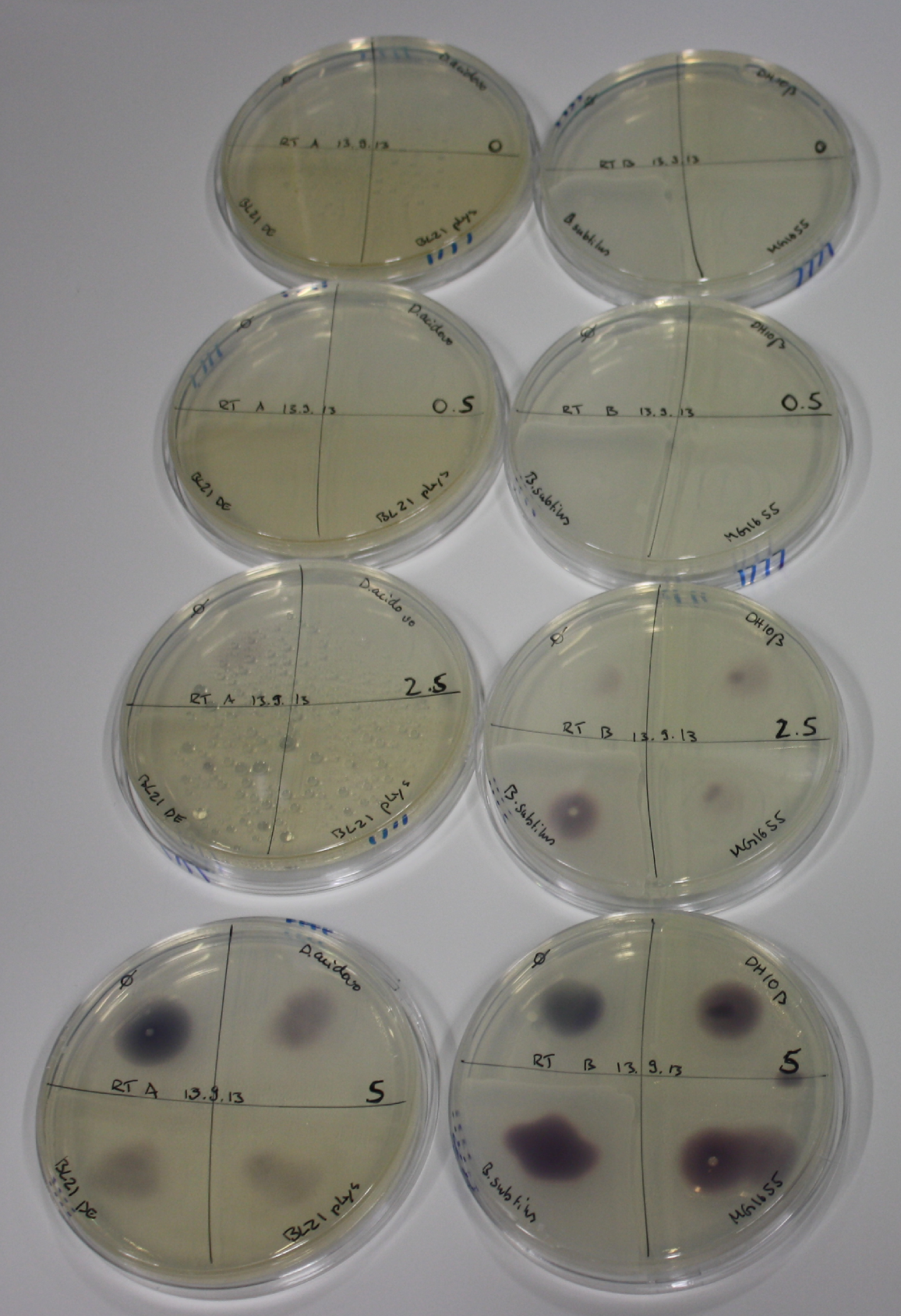
On Agar Plate following 3 Day Incubation at RT
- Media was chelex treated, agar added and autoclaved, before plates were poured.
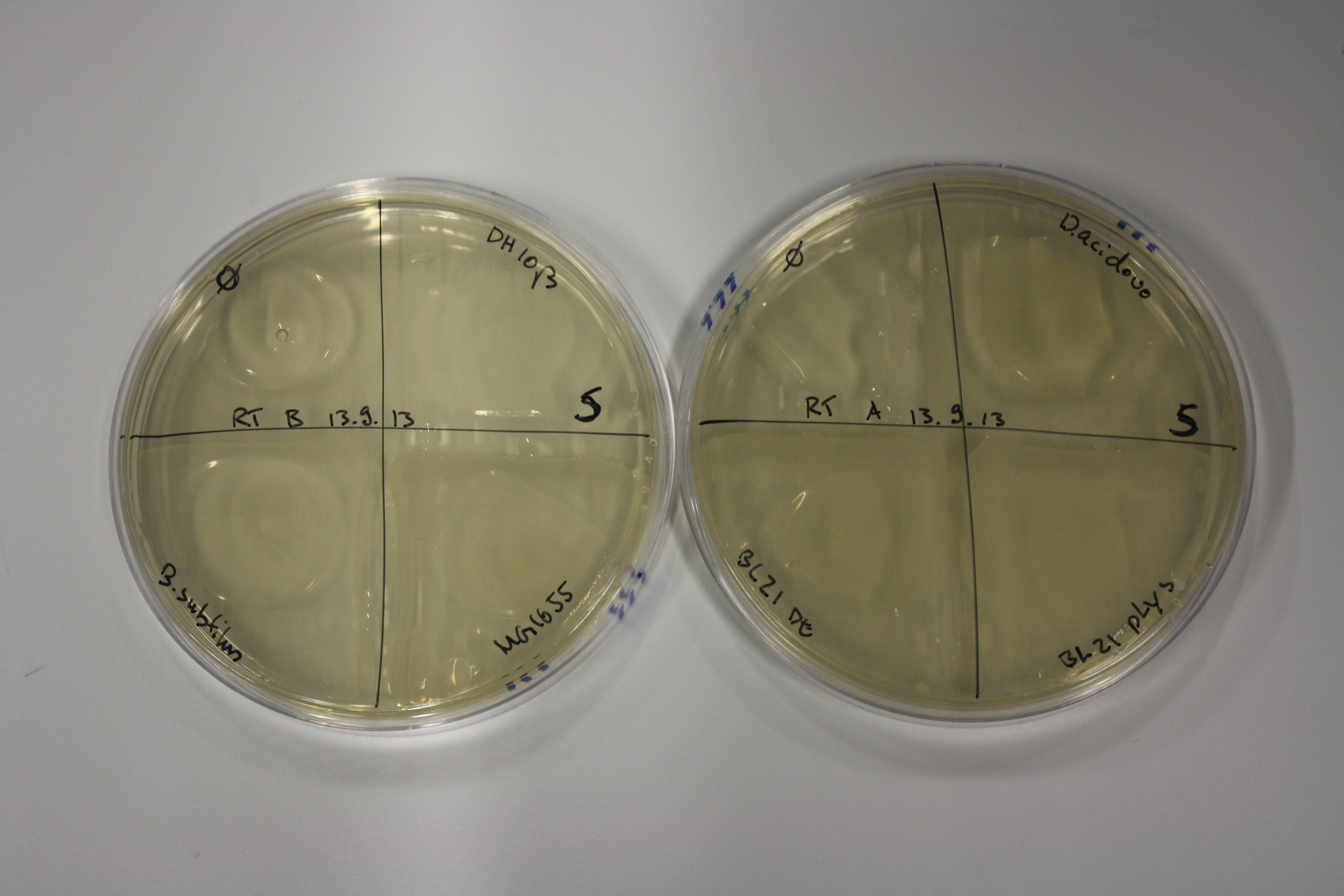
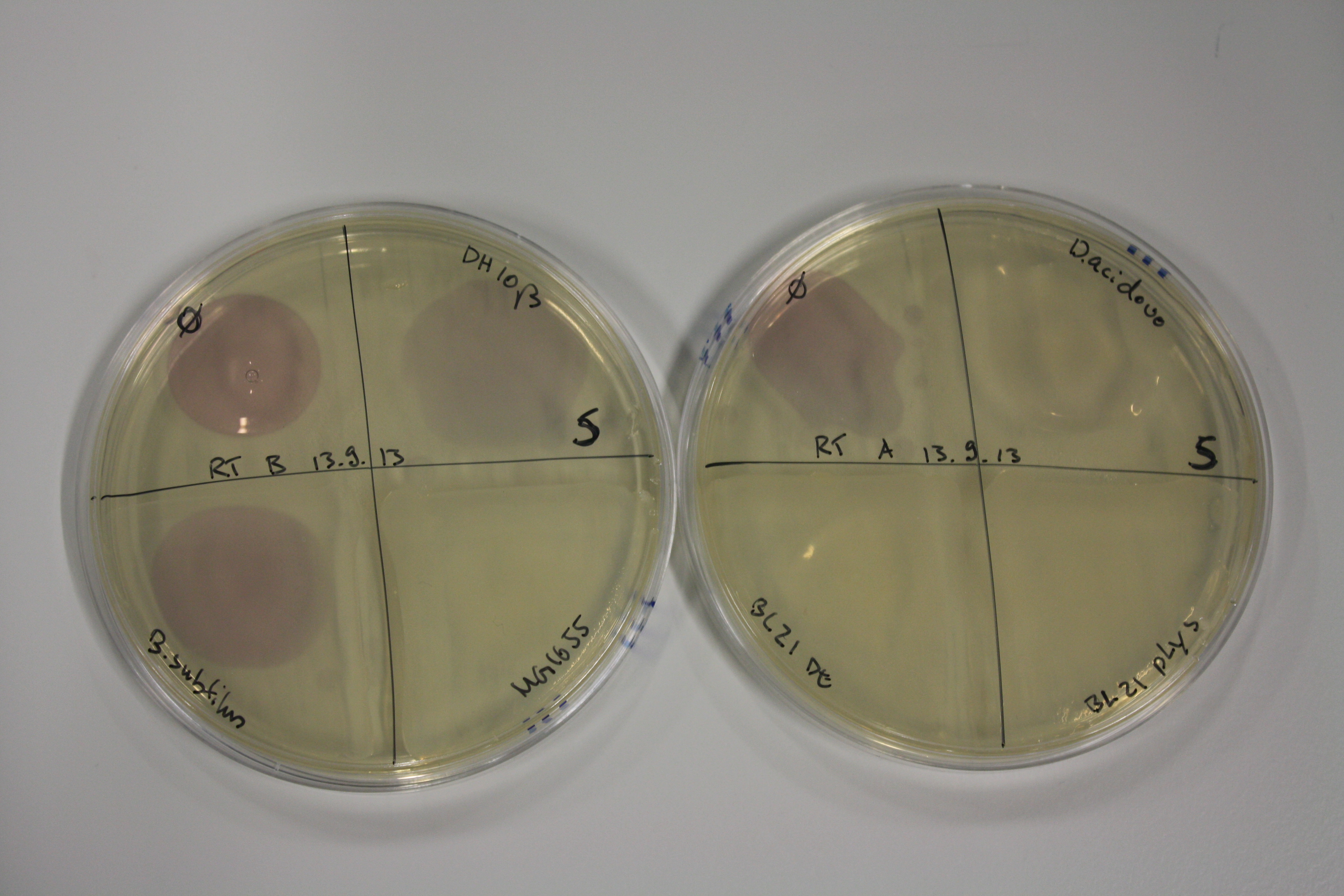
- On agar plates, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 and Bacillus subtilus were plated from liquid culture and grown for three days at RT.
- 14.4 ml soft agar (0.5%) was mixed with gold chloride [100 g/l] as follows and 200 µl per quarter plate added:
| Final gold chloride concentration [mM] | Volume goldchloride stock [µl] |
|---|---|
| 0 | 0 |
| 0.5 | 2.7 |
| 2.5 | 13.5 |
| 5 | 27 |
Result
| Agar plate | Goldchloride [mM] | H2O | D.acidovorans | BL21 DE3 | BL21 pLys | H2O | DH10ß | B.circulans | MG1655 |
|---|---|---|---|---|---|---|---|---|---|
| ACM | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| ACM | 0.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
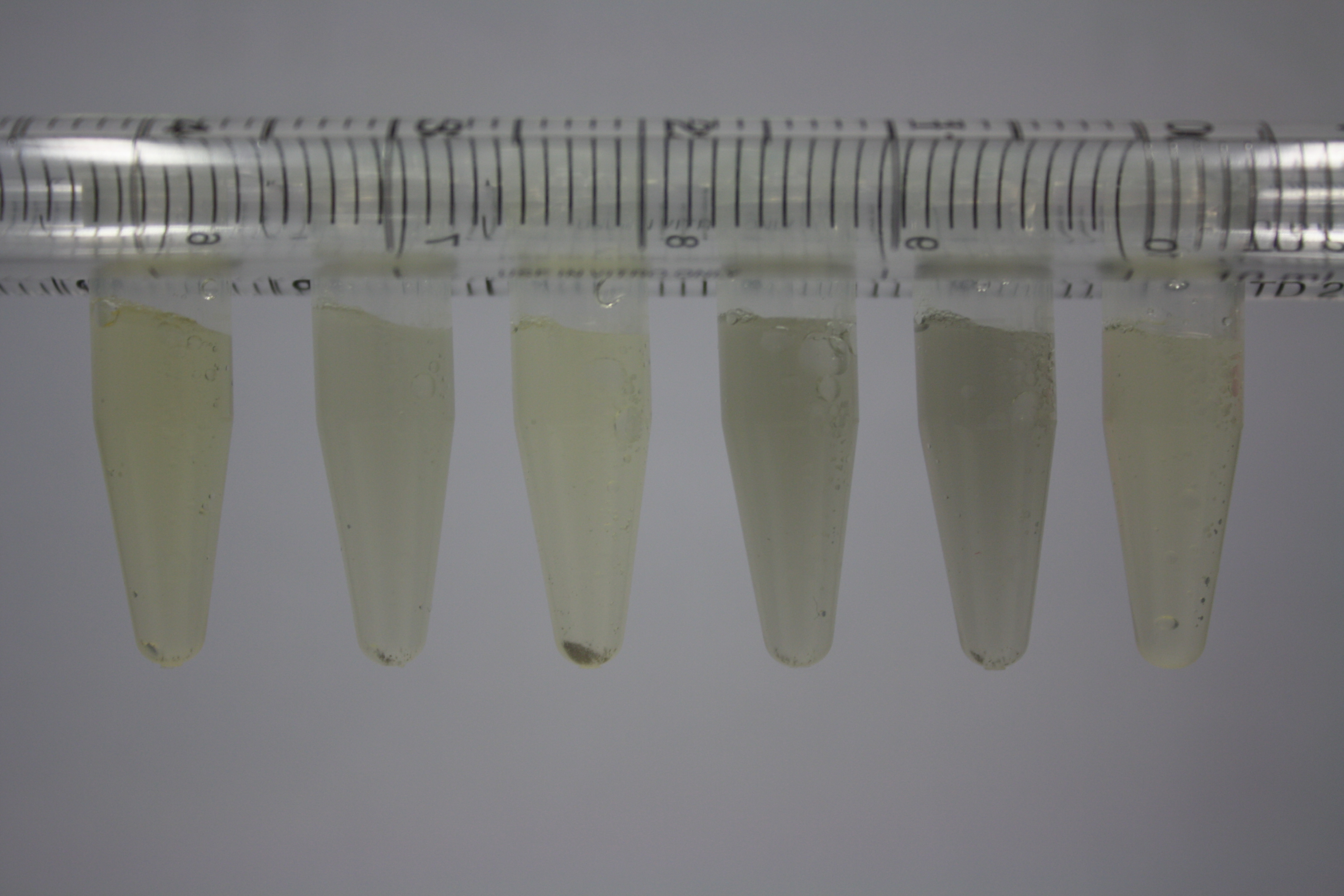
| ACM | 2.5 | no reaction | no reaction | no reaction | no reaction | minor coloring | minor coloring | intermediate coloring | minor coloring |
| ACM | 5 | strong coloring | strong coloring | minor coloring | minor coloring | intermediate coloring | strong coloring | strong coloring | strong coloring |
| LB | 5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 5 | strong coloring | strong coloring | no reaction | no reaction | strong coloring | strong coloring | strong coloring | no reaction |
On ACM plates following RT incubation, D.acidovorans and the neighbouring negative control, B.circulans and E.coli MG1655 show minor immediate coloring at 5 mM. At lower concentrations, no activity can be observed.
- => There is less Delftibactin expression in D.acidovorans at lower temperatures.
- => E.coli BL21 pLys remains a promissing candidate for expression since they show the least background activity.
On LB plates following RT incubation, there is again nothing to see.
On M9 plates following RT incubation, there is again high background at negative controls as well as intermediate coloring for D.acidovorans and B.circulans.
We will therefore analyze gold precipitation capacities of supernatants of D.acidovorans, E.coli DH10ß, BL21 DE3, BL21 pLys and MG1655 in ACM liquid medium following 48 and 72 h.
In ACM Medium following 2 Day Incubation at 30°C
- In 5 ml ACM medium, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 and pure medium were inocculated and grown for two days at 30°C.
- 1 ml supernatant was mixed with gold chloride [100 g/l] as follows:
| Final gold chloride concentration [µg/ml] | Volume goldchloride stock [µl] |
|---|---|
| 0.6 | 6 |
Result
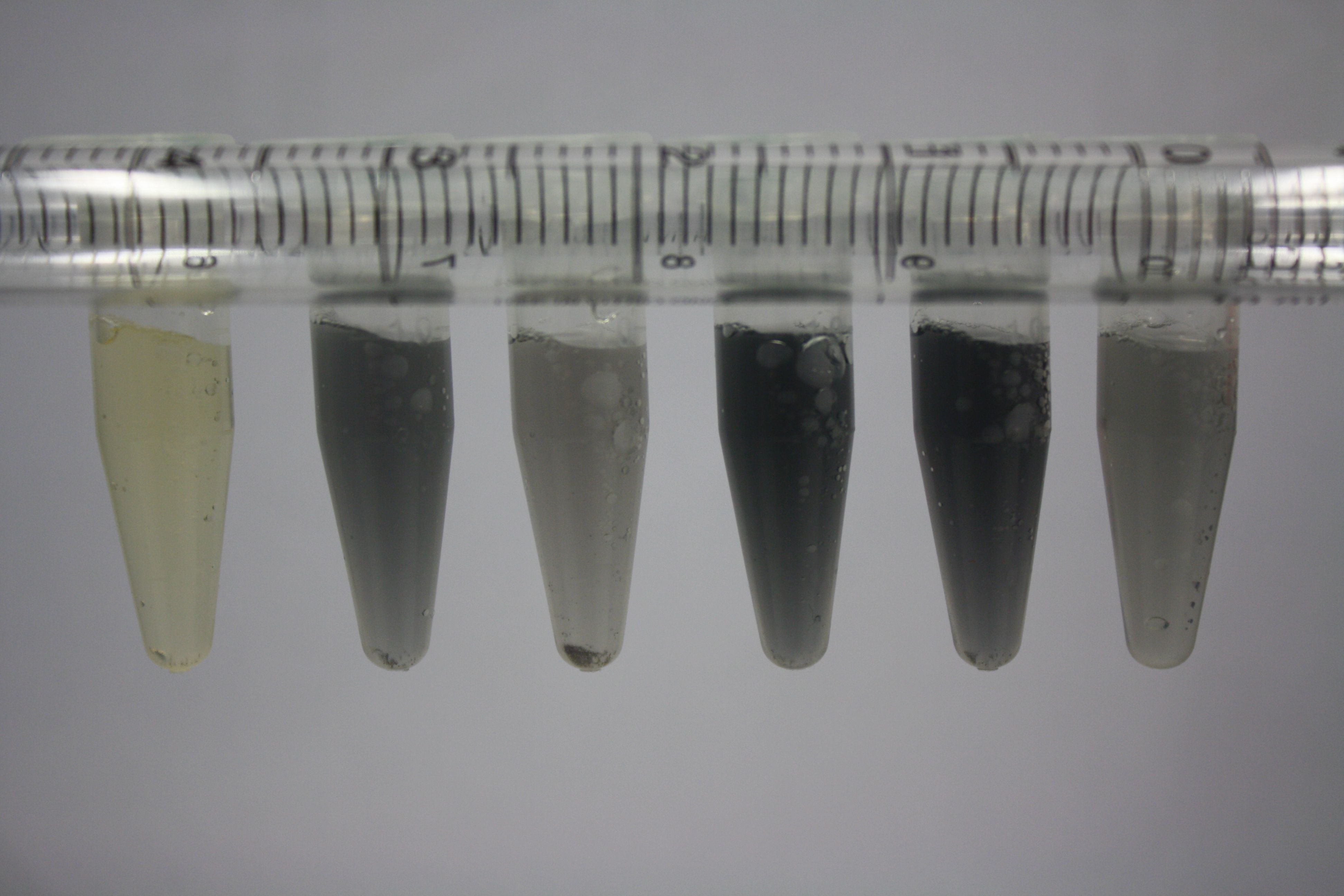
Following addition of gold chloride solution, E.coli DH10ß, Bl21 DE3, and BL21 pLys showed rapid purple-black coloring indicating gold nanoparticle formation. They exceed the capacity of D.acidovorans, which showed gold precipitation slightly faster than E.coli MG1655. The ACM did not show any background activity.
We will extend the incubation time of the cultures further to evaluate differences, possibly due to accumulation of Delftibactin in the medium.
In ACM Medium following 3 day Incubation at 30°C
- In 5 ml ACM medium, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 were inocculated and grown for three days at 30°C.
- 1 ml supernatant was mixed with gold chloride [100 g/l] as follows:
| Final gold chloride concentration [µg/ml] | Volume goldchloride stock [µl] |
|---|---|
| 0.6 | 6 |
Result
Again, E. coli DH10ß, Bl21 DE3, and BL21 pLys showed rapid purple-black coloring exceeding the capacity of D.acidovorans and E. coli MG1655. These results are highly inconclusive and the experiment has to be repeated.
Purification of Delftibactin
Purification
- Grew 1 L of D. acidovorans SPH1 for 24h in ACM media at 30°C. (2x 500 mL ACM 15 mL about 3 days old D. acidovorans culture in ACM)
- Centrifugation of culture at 3750 rpm for 30 min
- Retrieved supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 400 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
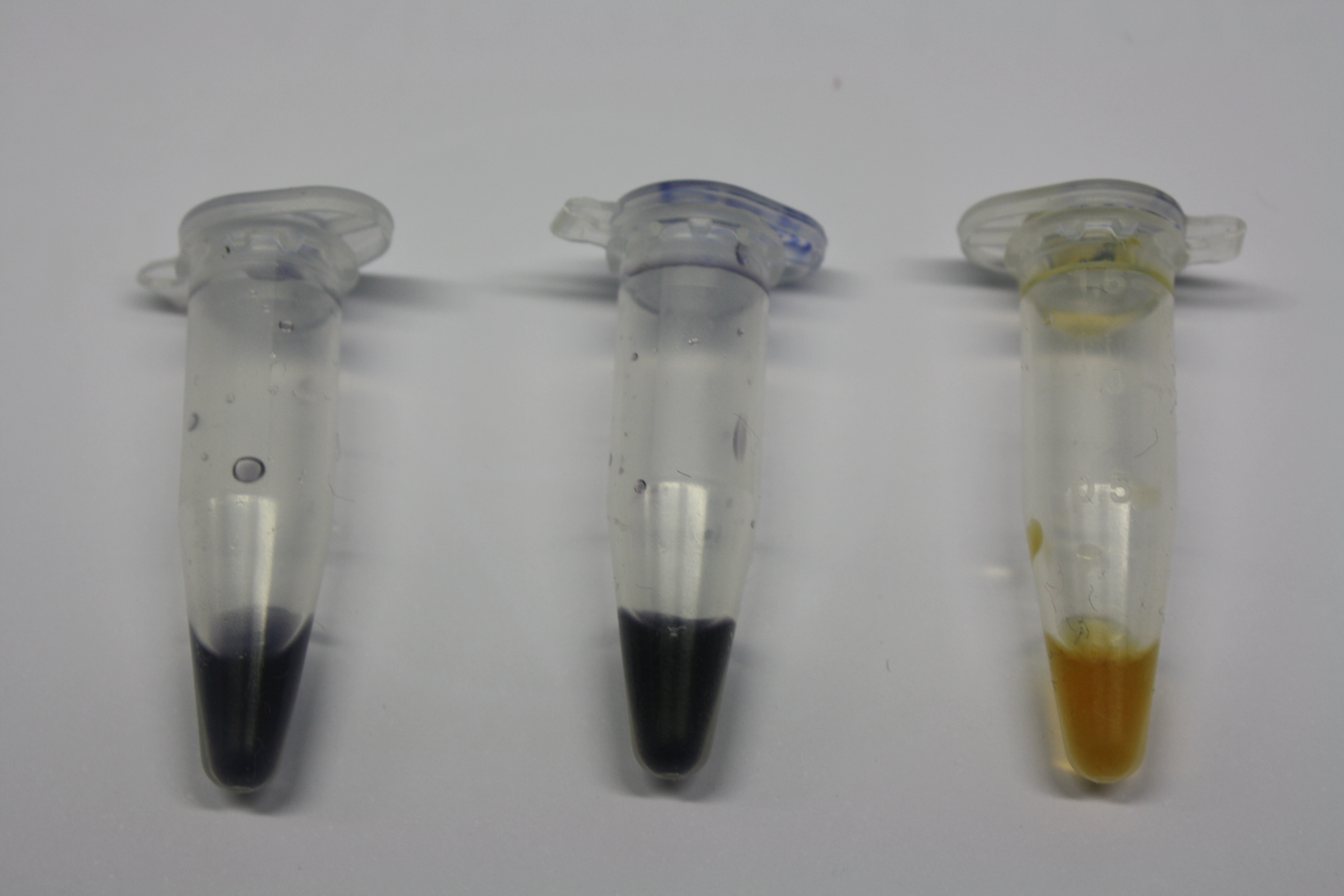
Testing Purified Delftibactin
Different intermediate products were tested for their ability to precipitate gold. For this, 3 µl of AuCl3 (100g/l) were added to 500 µl solution. The outcome is presented in the following table.
| Sample | Precipitating Gold? | Colour of Precipitate | Time until Precipitation Started |
|---|---|---|---|
| AMC media | yes | grey | ~2 h |
| Supernatant of D. acidovorans SPH-1 culture | yes | black | 1:30 min |
| Supernatant after treatment with HP20 | yes | black | 1:40 min |
| HP20 after elution step | no | -- | -- |
| Methanol | no | -- | -- |
| Methanol used for elution | no | -- | -- |
| Sample | Diluted in Water | Volume sample | Gold Solution 100g/l | Precipitating gold? | Colour of Precipitate | Time until Precipitation Started |
|---|---|---|---|---|---|---|
| ACM media | -- | 160 µl | 1 µl | yes | black | 1 h |
| Supernatant of D. acidovorans in ACM, filtered | -- | 160 µl | 1 µl | yes | black | 1 h |
| Purified Delftibactin after dilution in 50:50 Methanol, Water | -- | 160 µl | 1 µl | no | -- | -- |
| Water | -- | 250 µl | 1.5 µl | no | -- | -- |
| ACM media | 1:10 | 250 µl | 1.5 µl | no | -- | -- |
| Supernatant of D. acidovorans in ACM | 1:10 | 250 µl | 1.5 µl | no | -- | -- |
| Supernatant of D. acidovorans in ACM, filtered | 1:10 | 250 µl | 1.5 µl | no | -- | -- |
| Purified Delftibactin after dilution in 50:50 Methanol, H2O | 1:10 | 250 µl | 1.5 µl | yes | gray | over night |
Micro-TOF
The supernatant of a 24 h D. acidovorans SPH-1 culture was analysed before and after the purification procedure using a MICRO-TOF. As negative control unpurified ACM media was measured as well.
File:Heidelberg 20130911Malditof.pdf
Result
Delftibactin was clearly present in the purified supernatant of the D. acidovorans SPH-1 culture. In contrast it could not be detected in the ACM media or the supernatant which was not purified.
Triple Clone pIK8.6, DelH and DelH I 6B
Preparations
Electrocompetent E. coli DH10ß and BL21 DE3 were prepared as described.
Electroporation
We electroporated all three plasmids into E. coli DH10ß using pIK8.6. Although the DelH clone I 6B has a deletion in the ribosome binding site, we hope that we still get enough expression of DelH.
| Electroporation | Total amount of DNA [ng] | Ratio pIK2.6: DelH:DelRest | pIK8.6-1(12 Kb, 146 ng/µl) | DelH-I6B midi (23 Kb, 235.2 ng/µl) | DelRest (32 Kb, 2.4 µg/µl) |
|---|---|---|---|---|---|
| Triple into E. coli DH10ß | 774.2 | 1:2:3 | 1 µl of 1:10 (146 ng) | 1 µl (235.2 ng) | 1.5 µl of 1:10 (375 ng) |
Preparation of Cultures for Purification and MICRO-TOF
| Bacteria | Media | Antibiotics |
|---|---|---|
| -- | ACM | -- |
| E. coli DH10ß | ACM | -- |
| Delftia acidovorans SPH-1 | ACM | -- |
| E. coli DH10ß with plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 | ACM | Ampicillin, Chloramphenicol, Kanamycin |
- Inoculation of 10 ml of over night cultures of each sample
- Growth at 30°C (Delftia acidovorans) and 37°C (E. coli DH10ß and E. coli DH10ß + plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6)
- For each culture, 500 ml of ACM media with antibiotics was inoculated with the pre-cultures
- The cultures were grown for about 8 h.
ODs
- E. coli DH10ß = 1.2
- Delftia acidovorans SPH-1 = 0.4
- E. coli DH10ß + plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 = 0.8
Purification of Delftibactin
- Centrifugation of cultures at 3750 rpm for 30 min
- Retrieved supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 200 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
MICRO-TOF
File:Heidelberg Microtof0913.pdf
Result
Apparently the purification of delftibactin did not work, as even in the positive control (D. acidovorans SPH-1) no delftibactin was detected. As the cultures were harvested after only a short time of growth it is suspected that maybe the production of delftibactin had not started yet. The next time the cultures should grow for longer.
17-09 - 22-09-13
Triple Clone pIK2.6, DelH and DelH I 6B
Electroporation
We electroporated all three plasmids again into E. coli BL21 DE3. This time, we incubated them them for 3 full days.
| Electroporation | Total amount of DNA [ng] | Ratio pIK8.6: DelH:DelRest | pIK8.6-1(12 Kb) | DelH-I6B midi (23 Kb, 235.2 ng/µl) | DelRest (32 Kb, 2.4 µg/µl) |
|---|---|---|---|---|---|
| Triple into E. coli DH10ß | 774.2 | 1:2:3 | 1 µl of 1:10 (146 ng) | 1 µl (235.2 ng) | 1.5 µl of 1:10 (375 ng) |
Preparation of Cultures for Purification and MICRO-TOF
| Bacteria | Media | Antibiotics |
|---|---|---|
| -- | ACM | -- |
| Delftia acidovorans SPH-1 | ACM | Ampicillin |
| E. coli BL21 DE3 | ACM | -- |
| E. coli BL21 DE3 with plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 | ACM | Ampicillin, Chloramphenicol, Kanamycin |
- Inoculation of 10 ml of over night cultures of each sample
- For DH10β with plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 no such culture was made
- Growth at 30°C
- For each culture 1 L of ACM media with antibiotics was inoculated with this pre-culture
- 1 L of ACM media with antibiotics was directly inoculated with 1 mL of a 3 hour culture DH10β with plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 which had originally been made from a clone which had been picked from the agar plate. The 1 L culture was started 3 hours later than the other cultures
- Expression was induced in E. coli BL21 DE3 with 1 mM IPTG after 8 h of growth
- Cultures were harvested after 64 hours of growth
Purification of Delftibactin
- Centrifugation of cultures at 3750 rpm for 30 min
- Retrieved supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 400 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
MICRO-TOF
File:Heidelberg Microtof0925.pdf
Result
Only in the culture of D. acidovorans SPH-1 Delftibactin could be detected. The samples were negative for delftibactin. Apparently our triple clone does not produce delftibactin. However, this is not surprising, since the DelH clone I 6B harbors a mutation in the promoter region.
23-09 - 29-09-13
Evaluation of Background Expression and Indcibility of Possible Target Strains
Electroporation of mRFP Controls
1 µl miniprpep DNA of the backbones pSB6A1 and pSB3C5 containing mRFP were elctroporated in E.coli Bl21 DE3. Cells were streaked on LB Amp + Chlor plates and stored at RT for two days.
Result
All plates were heavily overgrown. As discussed next, the obtained BL21 DE3 were actually BL21 DE3 pLys and therefore resitant to chloramphenicol. Electroporation has to be repeated, after real E. coli BL21 DE3 are obtained.
New BL21 DE3
We obtained new BL21 DE3 from the Russel Lab, which were obtained from NEB directly. Cells were inocculated in 10 ml LB and streaked on LB Chlor plates. The vial was frozen again at -80°C.
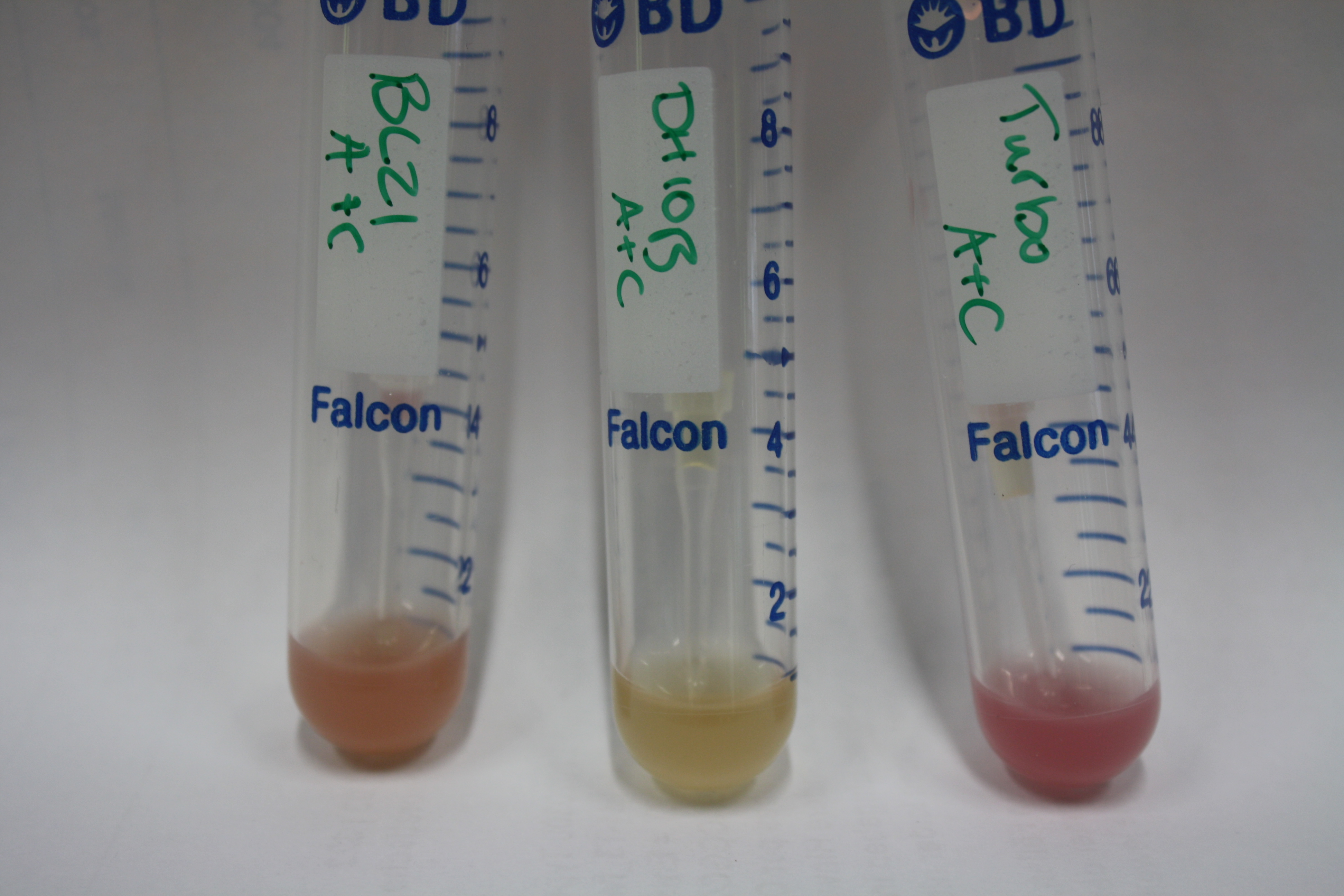
Electroporation of mRFP Controls
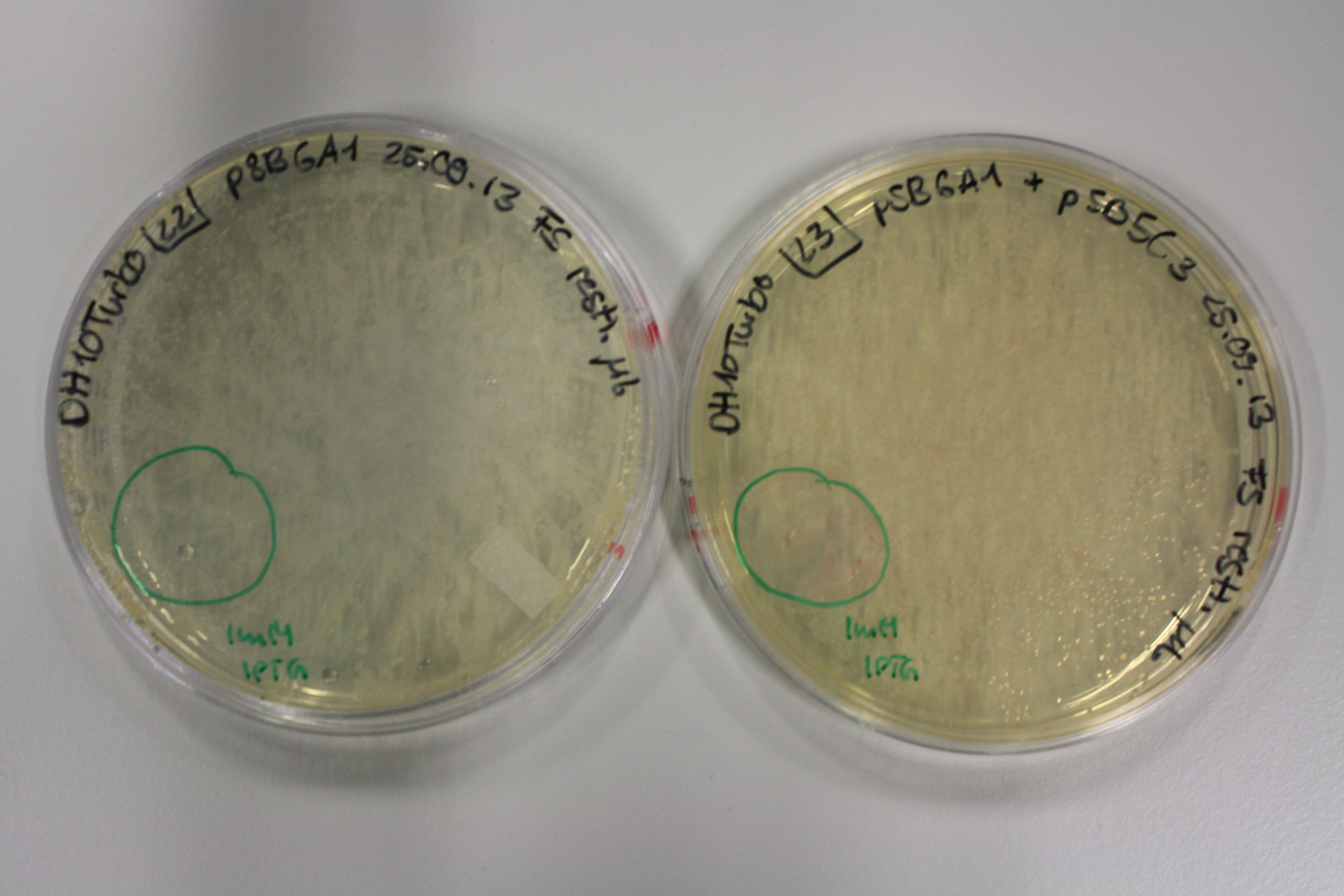
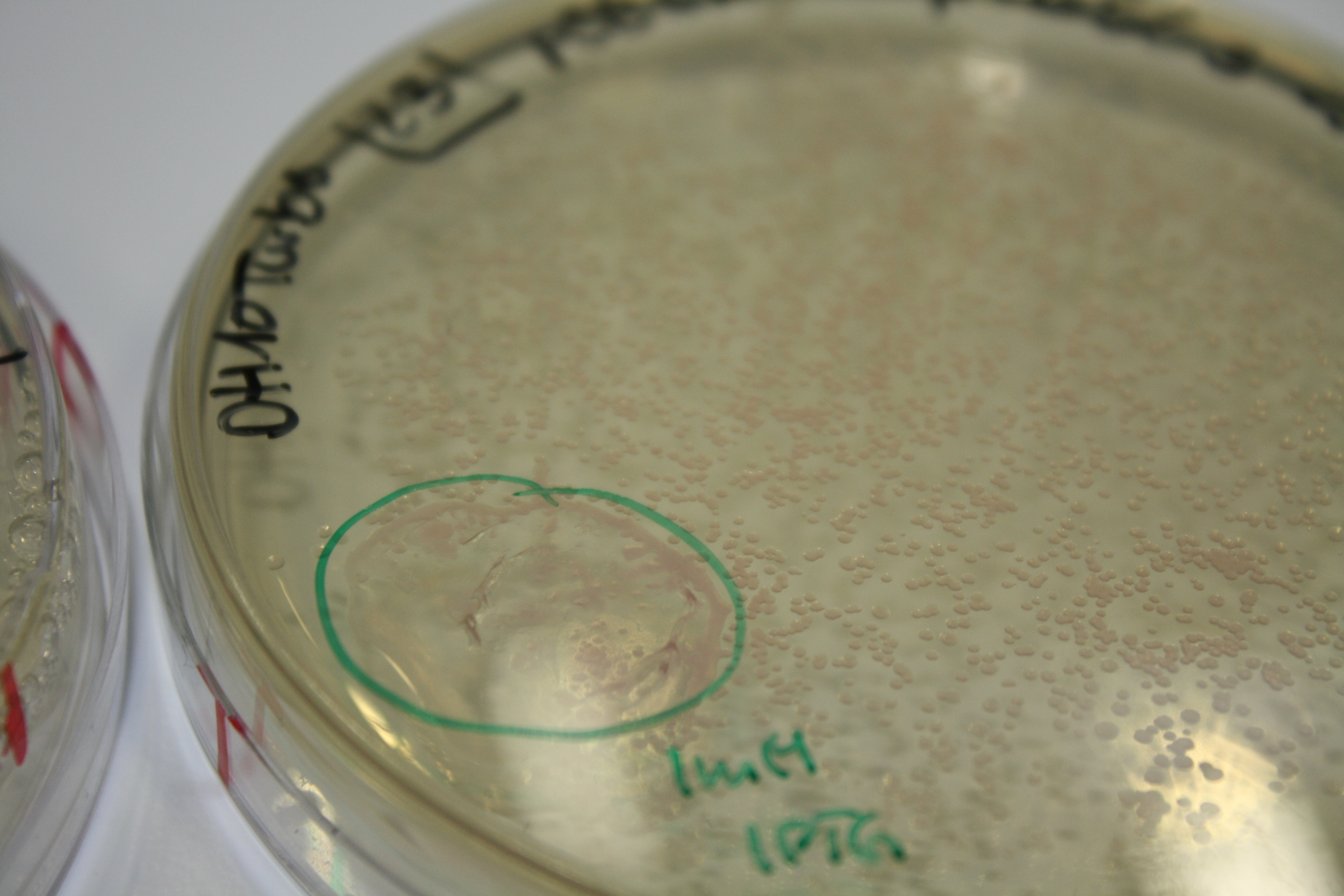
Real E. coli BL21 DE3, DH10ß and NEB Turbo were co-electroporated with 1 µl miniprpep DNA of the backbones pSB6A1 and pSB3C5 containing mRFP to check for leaky ecpression. Transformed bacteria were streaked on 2YT plates with Amp + Chlor and incubated ON at 30°C.
The next day, expression of mRFP on backbones pSB6A1 and pSB3C5 was induced by 50 µl 1 mM IPTG as well as inocculated in YT Amp + Chlor + 1 mM IPTG.
Result
After 6 h, only BL21 DE showed expression of mRFP. Yet, it was not restricted to the induced area. Another 10 h later, all strains showed red colonies with and without induction. E. coli BL21 DE3 showed least background expression and intermediate expression upon induction on plate and in liquid media.
Electrocompetent E. coli BL21 DE + DelRest and pIK8.6
Electroporation of DelRest, DelH and pIK8.6
As stated above, BL21 DE3 showed least background expression of mRFP and were therefore chosen for preparation of electrocompetent cells as described. Cells were streaked on 2YT Kan + Chlor plates and incubated at RT for 2 days.
Colony-PCR Conditions CP.W22.A
6 colonies were analyzed for Del Rest and pIK8.6 by colony PCR.
- DelL
| Reagent | Amount [µl] |
|---|---|
| Colonies | - |
| FS_14 10 µM | 1 |
| FS_15 10 µM | 1 |
| OneTaq Master Mix | 10 |
| DMSO | 1 |
| ddH2O | 7 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 12 | 95 | 30 |
| 66 ↓ 0.5 | 30 | |
| 72 | 90 | |
| 18 | 95 | 30 |
| 63 | 30 | |
| 72 | 90 | |
| 1 | 72 | 720 |
| 1 | 12 | inf |
- pIK8.6
| Reagent | Amount [µl] |
|---|---|
| Colonies | - |
| VF2 10 µM | 1 |
| IK_25 10 µM | 1 |
| OneTaq Master Mix | 10 |
| DMSO | 1 |
| ddH2O | 7 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 35 | 95 | 30 |
| 54 | 30 | |
| 72 | 90 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result
Expected band: ~1.4 Kb (DelL) and ~1 Kb (pIK8.6)
All of the analyzed colonies shows expected band for DelL.
- => Inocculate 2 ml 2YT Kan + Chlor with 2 colonies for preparation of electrocompetent cells
Electrocompetent Cells
Electrocompetent E. coli BL21 DE3 + DelRest + pIK8.6 wer prepared as described.
30-09 - 06-10-13
Triple Clone pIK.6, DelRest and DelH C5
Electroporation
E. coli BL21 DE3 + DelRest + pIK8.6 were electroporated with DelH C5 miniprep DNA and streaked on 2YT + Amp + Chlor + Kan plates and incubated at RT for 2 days.
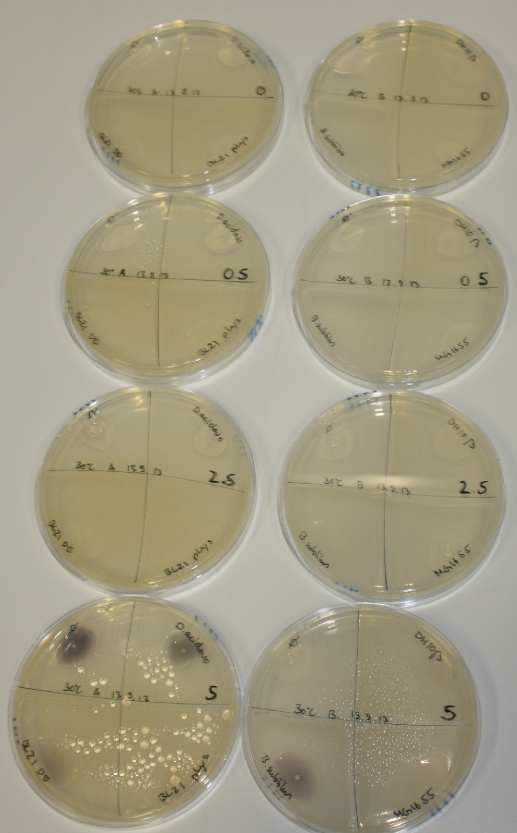
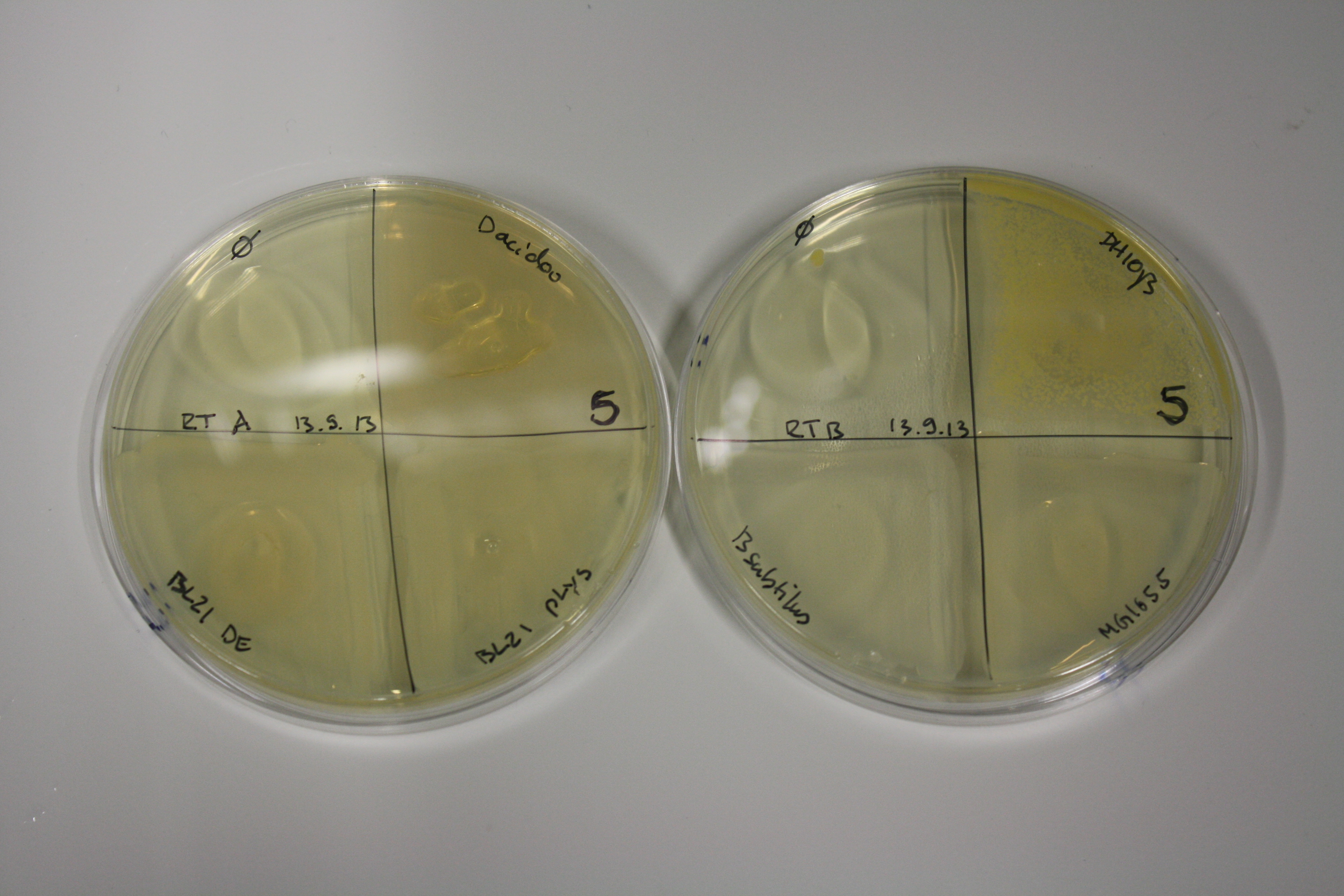
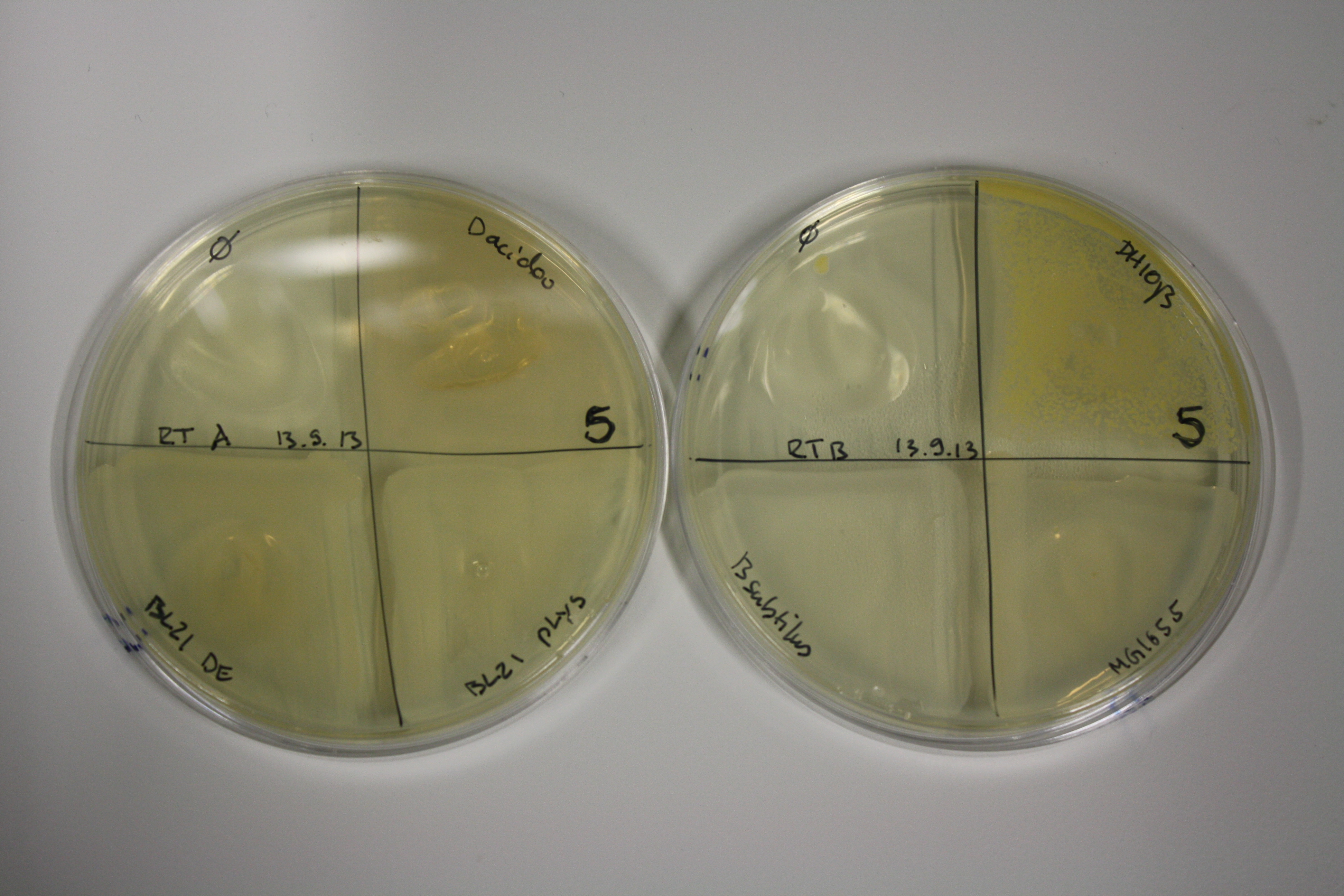
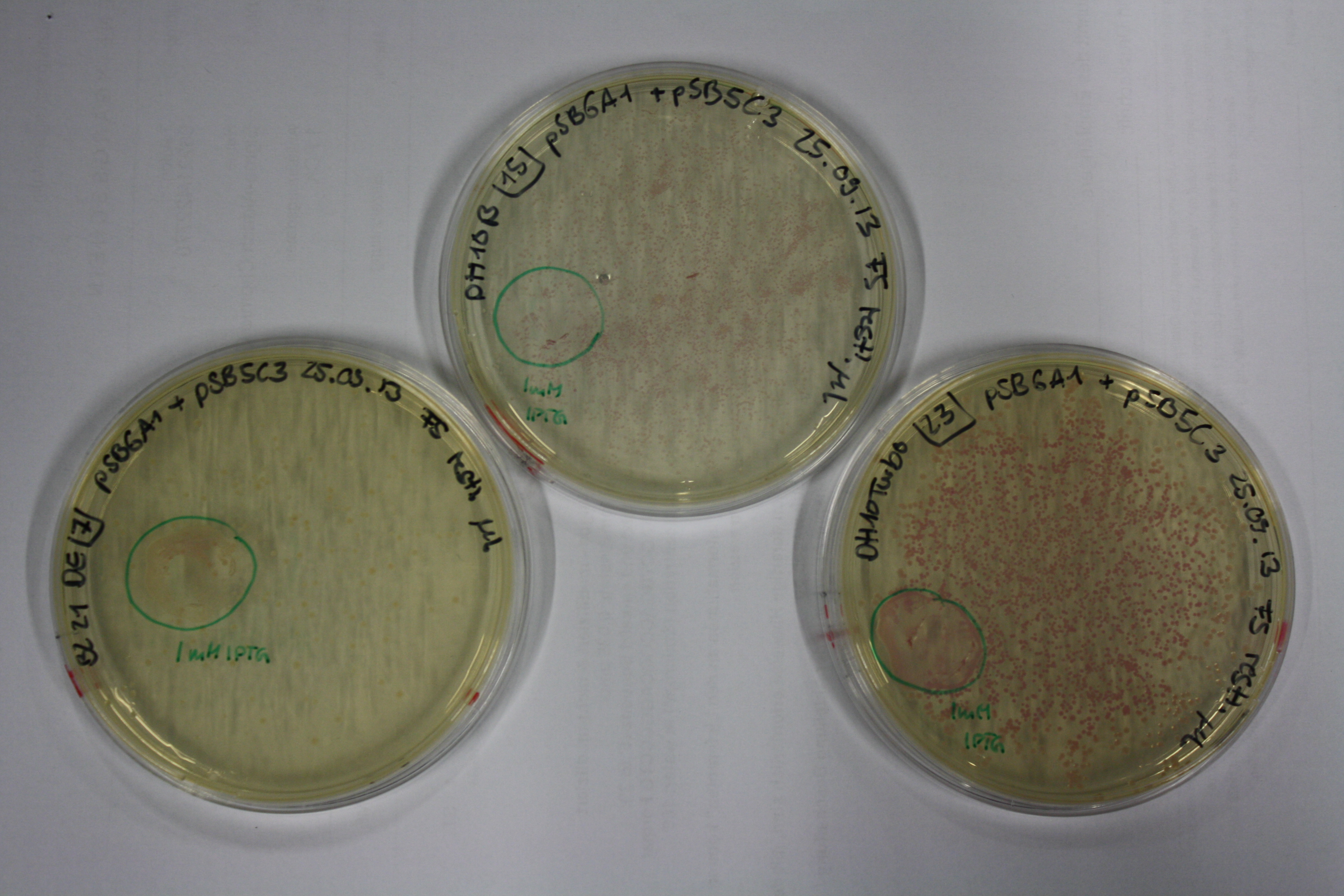
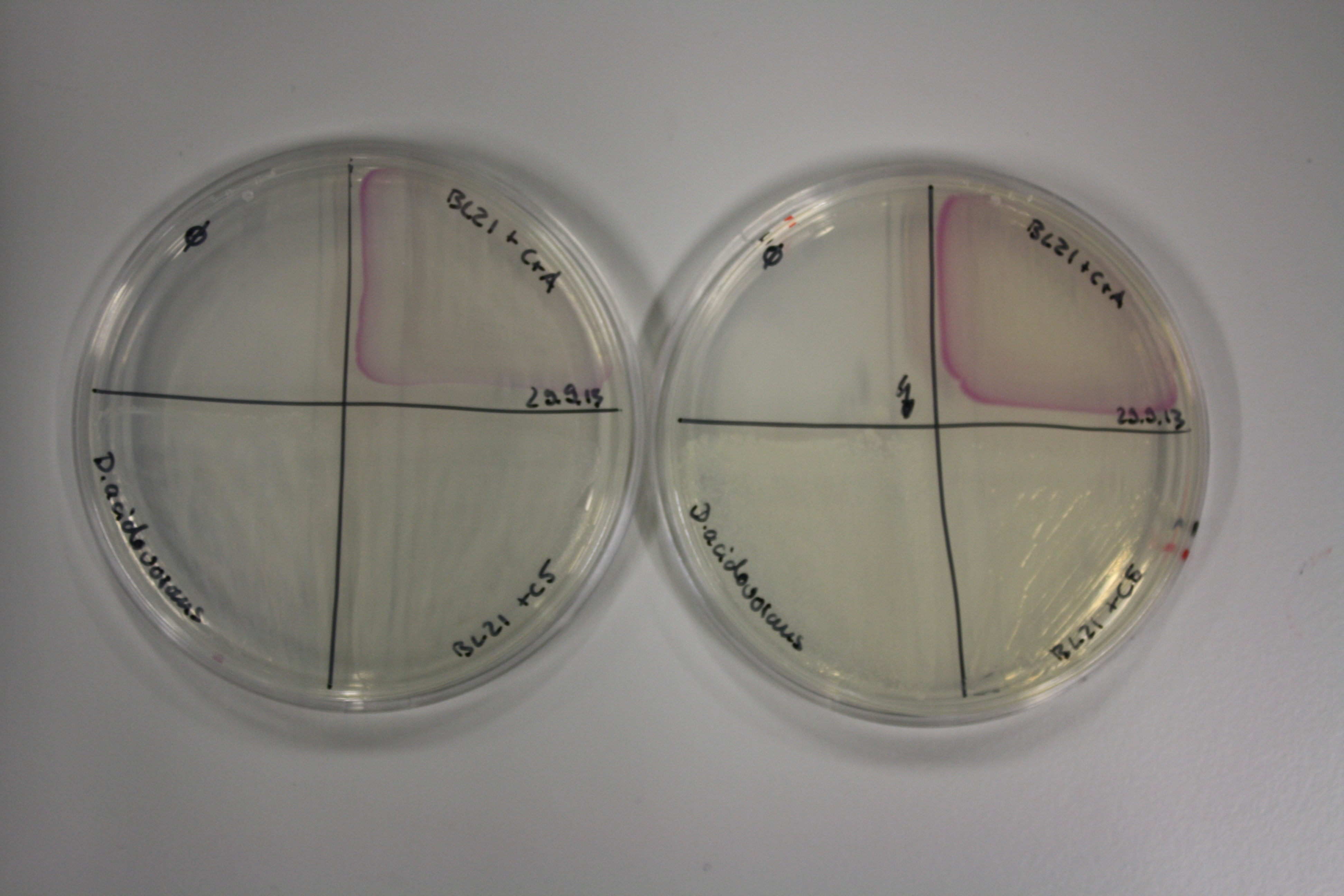
Evaluation of Gold Precipitation on ACM Plates
In order to evaluate gold nanoparticle formation capacity DelH clone C5 in presence of DelRest and pIK8.6, they were streaked on ACM plates as follows and incubated at 30°C for 3 days. As controls, we used the BL21 DE3 transformed with pSB3C5 and pSB6A1.
| Plate | Antibiotic | Cells |
|---|---|---|
| ACM | - | D.acidovorans |
| E.coli BL21 DE3 + pSB3C5 + pSB6A1 | ||
| E.coli BL21 DE3 + DelH C5 + DelRest + pIK8.6 | ||
| empty | ||
| ACM | Amp + Chlor + Kan | D.acidovorans |
| E.coli BL21 DE3 + pSB3C5 + pSB6A1 | ||
| E.coli BL21 DE3 + DelH C5 + DelRest + pIK8.6 | ||
| empty | ||
| ACM + IPTG | Amp + Chlor + Kan | D.acidovorans |
| E.coli BL21 DE3 + pSB3C5 + pSB6A1 | ||
| E.coli BL21 DE3 + DelH C5 + DelRest + pIK8.6 | ||
| empty |
Following 3 day incubation, 200 µl 5 mM gold chloride in soft agar was applied.
Result
Unexpectedly, the D.acidovorans control grew on the ACM + Amp + Chlor + Kan plates, indicating a contamination with triple resistant bacteria. Therefore, the experiment is inconclusive and has to be repeated. Moreover, the background control shows significant activity.
Nevertheless, there was neither increased activity of the induced 'E.coli BL21 DE3 containing DelH C5, DelRest and pIK8.6 compared to the E.coli BL21 DE3 negative control nor to the not induced control.
Preparation of Cultures for Purification and MICRO-TOF
| Bacteria | Media | Antibiotics |
|---|---|---|
| E. coli BL21 DE3 | ACM | -- |
| Delftia acidovorans SPH-1 | ACM | -- |
| E. coli BL21 DE3 with plasmids pHM04 (C5), pFSN (D8w) & pIK8.6 | ACM | Ampicillin, Chloramphenicol, Kanamycin |
- Inoculation of 10 ml of over night cultures of each sample
- Growth at 30°C
- For each culture, 1 L of ACM media with antibiotics was inoculated with the pre-culture
- Expression was induced in all E. coli BL21 DE3 with 1 mM IPTG after 24 h of growth
- All cultures were supplemented with 10 mM sodium propionate after 24 h of growth
- The cultures selected with ampicillin were supplemented with an additional 1:2000 ampicillin after 24 h of growth
- Cultures were harvested after 48 hours of growth and 24 hours after induction
Purification of Delftibactin
- Centrifugation of cultures at 3750 rpm for 45 min
Protocol for liquid culture
- Retrieved supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 400 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
Protocol for pellet
- Kept pellet
- Washed pellet with destilled water
- Resuspended pellet in 50 ml destilled water
- Freeze and thawed 8 times
- Centrifugation at 3750 rpm for 30 min
- Kept supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 400 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
MICRO-TOF
File:Heidelberg 20131004 MicroTOF.pdf
Result
Unexpectedly, the D. acidovorans positive control was negative for expression of Delftibactin. The results are therefore inconclusive and the experiment has to be repeated.
 "
"