Team:Paris Saclay/Notebook/August/8
From 2013.igem.org
(Created page with "{{Team:Paris_Saclay/incl_debut_generique}} ='''Notebook : August 8'''= <a id="section_modeling"> sdfsdfsd fsd fsd fds fsd fsd fsd sdfds ds fsd fd fsdf sd f d sdf fsd dfs </a> ...") |
(→2 - Electrophoresis to check the colony PCR products : BBa_K1155007) |
||
| (71 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
='''Notebook : August 8'''= | ='''Notebook : August 8'''= | ||
| - | + | =='''Lab work'''== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ==='''A - Aerobic/Anaerobic regulation system'''=== | |
| - | + | ===='''Obtaining biobricks in pSB3K3'''==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ====1 - Digestion of BBa_J04450 by EcoRI/PstI==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Nadia | |
| + | Used quantities : | ||
| + | * DNA : 5µL | ||
| + | * Buffer FD : 2µL | ||
| + | * EcoRI FD : 1µL | ||
| + | * PstI FD : 1µL | ||
| + | * H2O : 11µL | ||
| + | |||
| + | We incubate our digestion at 37°C for 1h30. | ||
| + | |||
| + | ====2 - Electrophoresis of BBa_J004450 digested by EcoRI/Pst1 to check if the digestion==== | ||
| + | Damir, Nadia | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel10808.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | *Well 1 : 6µL DNA Ladder | ||
| + | *Well 2 : 5µL of BBa_J004450 digested by EcoRI/Pst1 | ||
| + | *Gel : 0.8% | ||
| + | |} | ||
| + | |||
| + | Expected sizes : | ||
| + | |||
| + | *pSB3K3 : 2750kb | ||
| + | *GFP : 1069kb | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtained fragments at the right size. We will purify the highest band which contains the pSB3K3 plasmid. | ||
| + | |} | ||
| + | |||
| + | ====3 - Electrophoresis of BBa_J004450 digested by EcoRI/PstI==== | ||
| + | |||
| + | Anaïs | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" | [[File:Psgel20808.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | *Well 1 : 6µL DNA Ladder | ||
| + | *Well 2 : 45µL of BBa_J004450 digested by EcoRI/Pst I | ||
| + | *Gel : 0.8% | ||
| + | |} | ||
| + | |||
| + | pSB3K3 : 2750bp | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtained fragments at the right size. We will purify the highest band which contains the pSB3K3 plasmid. | ||
| + | |} | ||
| + | |||
| + | ==== 4- Electroelution of pSB3K3 digested by EcoRI/PstI==== | ||
| + | |||
| + | Nadia | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/electro|Electroelution]] | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We lost our plasmid. We will do the digestion again. | ||
| + | |} | ||
| + | |||
| + | ===='''Objective : obtaining BBa_K1155007'''==== | ||
| + | |||
| + | ====1 - Colony PCR of BBa_K115007 in DH5α==== | ||
| + | |||
| + | Anaïs | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | Transformation of 08/07/13 works. we will make a PCR Colony. | ||
| + | |} | ||
| + | |||
| + | We took a single colony and resuspend in 10µL H2O. For each Biobrick, we did 25 PCR Colonies. | ||
| + | |||
| + | Used quantities : | ||
| + | |||
| + | PCR preparation mix for 25 different colonies including: | ||
| + | ** Oligo 44 : 3.5µL | ||
| + | ** Oligo 45 : 3.5µL | ||
| + | ** Buffer Dream Taq : 70µL | ||
| + | ** dNTP : 28µL | ||
| + | ** Dream Taq : 5µL | ||
| + | ** H2O : 590µL | ||
| + | |||
| + | PCR reaction: | ||
| + | * DNA : 2µL | ||
| + | * Mix :23µL | ||
| + | Total volume: 25µL | ||
| + | |||
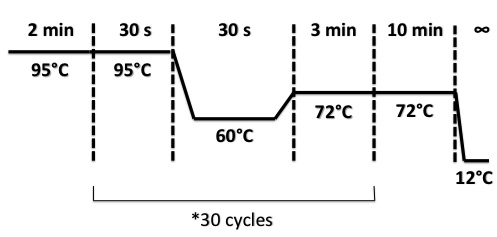
| + | PCR Program : | ||
| + | |||
| + | [[File:PsPcr808.jpg|400px]] | ||
| + | |||
| + | ====2 - Electrophoresis to check the colony PCR products : BBa_K1155007==== | ||
| + | |||
| + | Anaïs, Damir | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel30808.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 1 : 6µL DNA Ladder | ||
| + | * Well 2 to 24 : 10µL of BBa_K1155007 + 2µL of 6X loading dye | ||
| + | * Well 25 : 6µL DNA Ladder | ||
| + | * Well 26 : 6µL DNA Ladder | ||
| + | * Well 27 : 6µL DNA Ladder | ||
| + | * Well 28 : 10µL of BBa_K1155007 + 2µL of 6X loading dye | ||
| + | *Gel : 0.8% | ||
| + | |} | ||
| + | |||
| + | Expected size : | ||
| + | * BBa_K1155007 : 3583 bp | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtained fragments at the right size for colonies 10, 14 and 15. We will extract BBa_K1155007. | ||
| + | |} | ||
| + | |||
| + | ===='''Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== | ||
| + | |||
| + | ===='''1 - Tranduction of Km in MG1655Z1 ==== | ||
| + | |||
| + | Anaïs, Nadia | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | We didn't obtain colonies from the transduction of 08/07/13. We will do it again. | ||
| + | |} | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/transduction|Transduction]] | ||
| + | |||
| + | Our Mutant strain is BW1328 (Δfnr::Km) and the wild type strain is MG1655Z1. | ||
| + | |||
| + | We did the first step of the protocol : bacteriophage stock on BW1328 (Δfnr::Km). | ||
| + | |||
| + | ==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== | ||
| + | |||
| + | ===='''Objective : obtaining FNR and BphR2 proteins'''==== | ||
| + | |||
| + | ====1 - Extraction of plasmid pSB1C3 containing BphR2 ,FNR and RBS-FNR in competent cell DH5α==== | ||
| + | |||
| + | Damir | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | Transformation of 08/02/13 works. We will extract plasmids. | ||
| + | |} | ||
| + | |||
| + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We lost our plasmids. We will do the Gibson assembly again. | ||
| + | |} | ||
| + | |||
| + | ====2 - Gel purification of RBS-BphR2 Part I==== | ||
| + | |||
| + | Nadia, XiaoJing | ||
| + | |||
| + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We lost fragment. We will do the PCR again. | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| border="1" align="center" | ||
| + | |[[Team:Paris Saclay/Notebook/August/7|<big>Previous day</big>]] | ||
| + | |||
| + | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| + | |||
| + | |[[Team:Paris Saclay/Notebook/August/9|<big>Next day</big>]] | ||
| + | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 01:26, 5 October 2013
Notebook : August 8
Lab work
A - Aerobic/Anaerobic regulation system
Obtaining biobricks in pSB3K3
1 - Digestion of BBa_J04450 by EcoRI/PstI
Nadia
Used quantities :
- DNA : 5µL
- Buffer FD : 2µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- H2O : 11µL
We incubate our digestion at 37°C for 1h30.
2 - Electrophoresis of BBa_J004450 digested by EcoRI/Pst1 to check if the digestion
Damir, Nadia
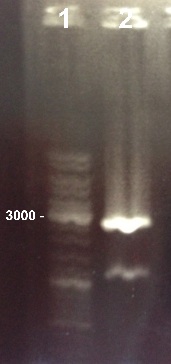
|
|
Expected sizes :
- pSB3K3 : 2750kb
- GFP : 1069kb
|
We obtained fragments at the right size. We will purify the highest band which contains the pSB3K3 plasmid. |
3 - Electrophoresis of BBa_J004450 digested by EcoRI/PstI
Anaïs
|
|
pSB3K3 : 2750bp
|
We obtained fragments at the right size. We will purify the highest band which contains the pSB3K3 plasmid. |
4- Electroelution of pSB3K3 digested by EcoRI/PstI
Nadia
Protocol : Electroelution
|
We lost our plasmid. We will do the digestion again. |
Objective : obtaining BBa_K1155007
1 - Colony PCR of BBa_K115007 in DH5α
Anaïs
|
Transformation of 08/07/13 works. we will make a PCR Colony. |
We took a single colony and resuspend in 10µL H2O. For each Biobrick, we did 25 PCR Colonies.
Used quantities :
PCR preparation mix for 25 different colonies including:
- Oligo 44 : 3.5µL
- Oligo 45 : 3.5µL
- Buffer Dream Taq : 70µL
- dNTP : 28µL
- Dream Taq : 5µL
- H2O : 590µL
PCR reaction:
- DNA : 2µL
- Mix :23µL
Total volume: 25µL
PCR Program :
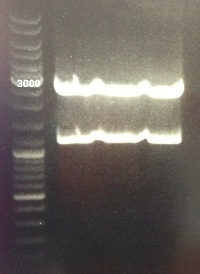
2 - Electrophoresis to check the colony PCR products : BBa_K1155007
Anaïs, Damir
Expected size :
- BBa_K1155007 : 3583 bp
|
We obtained fragments at the right size for colonies 10, 14 and 15. We will extract BBa_K1155007. |
Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - Tranduction of Km in MG1655Z1
Anaïs, Nadia
|
We didn't obtain colonies from the transduction of 08/07/13. We will do it again. |
Protocol : Transduction
Our Mutant strain is BW1328 (Δfnr::Km) and the wild type strain is MG1655Z1.
We did the first step of the protocol : bacteriophage stock on BW1328 (Δfnr::Km).
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
1 - Extraction of plasmid pSB1C3 containing BphR2 ,FNR and RBS-FNR in competent cell DH5α
Damir
|
Transformation of 08/02/13 works. We will extract plasmids. |
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
|
We lost our plasmids. We will do the Gibson assembly again. |
2 - Gel purification of RBS-BphR2 Part I
Nadia, XiaoJing
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
|
We lost fragment. We will do the PCR again. |
| Previous day | Back to calendar | Next day |
 "
"