Team:DTU-Denmark/Experiment1a
From 2013.igem.org
| (7 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{:Team:DTU-Denmark/Templates/StartPage| | + | {{:Team:DTU-Denmark/Templates/StartPage|Native anaerobic stability of nitrite in ''E. coli''}} |
__TOC__ | __TOC__ | ||
== Description == | == Description == | ||
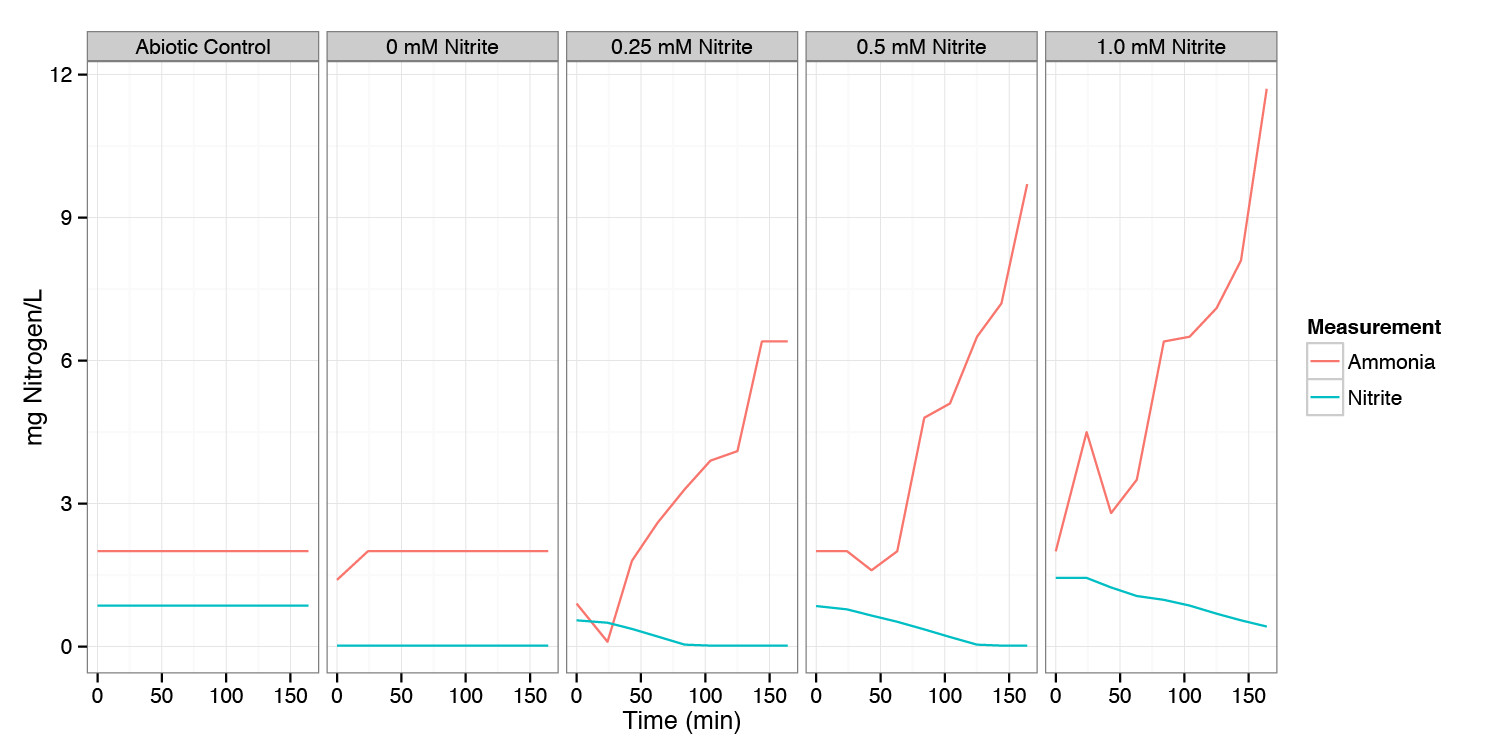
| - | The aim of | + | The aim of this experiment is to characterize the conversion of nitrite to ammonium in wild type ''E. coli'' under anaerobic conditions. |
| - | The experiment was performed by adding three different concentrations of nitrite to a medium with a growing native strain of ''E. coli''. The concentrations of NH<sub>4</sub><sup>+</sup> and NO<sub>2</sub><sup>-</sup> were measured every 20 minutes and the experiment lasted for 3 hours. Also | + | The experiment was performed by adding three different concentrations of nitrite to a medium with a growing native strain of ''E. coli''. The concentrations of ammonium (NH<sub>4</sub><sup>+</sup>) and nitrite (NO<sub>2</sub><sup>-</sup>) were measured every 20 minutes and the experiment lasted for 3 hours. Also two controls: with biomass only (no nitrite) and without biomass were analyzed in order to exclude any external influences on the nitrite conversion. |
== Methods == | == Methods == | ||
| - | + | The experiment was performed on [[Team:DTU-Denmark/Notebook/26_July_2013|July 26<sup>th</sup>]]. | |
| + | The procedure is based on the [https://2013.igem.org/Team:DTU-Denmark/Methods/Determining_concentration_of_nitrogen_compounds/Experiment_1a Experimental protocol]. | ||
== Results == | == Results == | ||
| Line 16: | Line 17: | ||
Mg of Nitrogen (as Nitrite or Ammonia) per litre measured for varying concentrations of Nitrite added at t=0. All experiments were performed anaerobically. Samples were measured every 20 min for 3 hours. | Mg of Nitrogen (as Nitrite or Ammonia) per litre measured for varying concentrations of Nitrite added at t=0. All experiments were performed anaerobically. Samples were measured every 20 min for 3 hours. | ||
| + | |||
| + | == Conclusions and Discussion == | ||
| + | |||
| + | Under anaerobic conditions, nitrite is consumed and converted to ammonium. Since we want to convert the nitrite to nitrous oxide, a functional prototype will require either that the pathway to nitrous oxide be designed to outcompete this pathway, or that this pathway that consumes nitrite be knocked out (and the transformants grown in a medium containing plentiful ammonium so they do not need to synthesize it). | ||
| + | |||
Latest revision as of 14:22, 4 October 2013
Native anaerobic stability of nitrite in E. coli
Contents |
Description
The aim of this experiment is to characterize the conversion of nitrite to ammonium in wild type E. coli under anaerobic conditions.
The experiment was performed by adding three different concentrations of nitrite to a medium with a growing native strain of E. coli. The concentrations of ammonium (NH4+) and nitrite (NO2-) were measured every 20 minutes and the experiment lasted for 3 hours. Also two controls: with biomass only (no nitrite) and without biomass were analyzed in order to exclude any external influences on the nitrite conversion.
Methods
The experiment was performed on July 26th. The procedure is based on the Experimental protocol.
Results
Mg of Nitrogen (as Nitrite or Ammonia) per litre measured for varying concentrations of Nitrite added at t=0. All experiments were performed anaerobically. Samples were measured every 20 min for 3 hours.
Conclusions and Discussion
Under anaerobic conditions, nitrite is consumed and converted to ammonium. Since we want to convert the nitrite to nitrous oxide, a functional prototype will require either that the pathway to nitrous oxide be designed to outcompete this pathway, or that this pathway that consumes nitrite be knocked out (and the transformants grown in a medium containing plentiful ammonium so they do not need to synthesize it).
 "
"