Team:Macquarie Australia/Safety
From 2013.igem.org
(Difference between revisions)
| (31 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{Team:Macquarie_Australia/Header}} | {{Team:Macquarie_Australia/Header}} | ||
| + | <html> | ||
| - | + | <style> | |
| - | < | + | li span { font-weight: normal; } |
| + | </style> | ||
<html> | <html> | ||
| + | <center><td><img src="https://static.igem.org/mediawiki/2013/thumb/2/29/Wiki_safety.jpg/599px-Wiki_safety.jpg" width=550 height=550></td> | ||
| + | </center> | ||
| + | <br><br> | ||
| - | |||
| - | |||
| - | + | <font size = 5><b><h1><center>iGEM Safety Questions</h1></font size><br><br> | |
| - | + | <font size = 3><center>1. Would any of your project ideas raise safety issues?</font></b><br><br> | |
| - | + | ||
| - | + | <font size = 4><b>Project Safety concerns</font size></b><br><br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <font size = 4><b>Project Safety concerns | + | |
To minimise risk of contamination or harm to researchers participating in the study or the environment, all students involved in the iGEM Macquarie University 2013 team received safety information according to the Macquarie University’s safety guidelines. The team also experienced an induction on how to operate specific machines within the lab, for example how to safely use the electroporator when dealing with chemicals and containers. We were supervised at all times by a senior member, or professor when working within the labs. A few examples of safe practices we employed to avoid biological material contamination include; disposal of biological waste in biohazard bins, correct labelling of materials, disinfect (with ethanol) the benches both before and after use and adhere to safe laboratory practices (including wearing lab coats, glasses and enclosed shoes etc.). Also to protect personnel and the environment, the chemicals involved in the lab work were used according to MSDS standards. <br><br> | To minimise risk of contamination or harm to researchers participating in the study or the environment, all students involved in the iGEM Macquarie University 2013 team received safety information according to the Macquarie University’s safety guidelines. The team also experienced an induction on how to operate specific machines within the lab, for example how to safely use the electroporator when dealing with chemicals and containers. We were supervised at all times by a senior member, or professor when working within the labs. A few examples of safe practices we employed to avoid biological material contamination include; disposal of biological waste in biohazard bins, correct labelling of materials, disinfect (with ethanol) the benches both before and after use and adhere to safe laboratory practices (including wearing lab coats, glasses and enclosed shoes etc.). Also to protect personnel and the environment, the chemicals involved in the lab work were used according to MSDS standards. <br><br> | ||
| Line 27: | Line 23: | ||
The host cells we used were Escherichia coli Star, which is categorised as a Biosafety Level 1 according to the World Health Organisation (WHO). Thus this E. coli strain has a low health risk to humans, the community and the environment. The plasmid pSB1S3 was provided by iGEM and is identified as a low safety risk (BSL1). The gBlocks, vector DNA and chlorophyll genes (which originated form Chalymodmonas reinhardtii) and were meticulously handled according to the gene regulations of the Australian Government’s Office of the Gene Technology Regulator, whom abide by the Gene Technology Act 2000.<br><br> | The host cells we used were Escherichia coli Star, which is categorised as a Biosafety Level 1 according to the World Health Organisation (WHO). Thus this E. coli strain has a low health risk to humans, the community and the environment. The plasmid pSB1S3 was provided by iGEM and is identified as a low safety risk (BSL1). The gBlocks, vector DNA and chlorophyll genes (which originated form Chalymodmonas reinhardtii) and were meticulously handled according to the gene regulations of the Australian Government’s Office of the Gene Technology Regulator, whom abide by the Gene Technology Act 2000.<br><br> | ||
| - | Pathogenic sequences? Search | + | Pathogenic sequences? Search <font size = 2> |
| + | <a class="three" href='http://www.ncbi.nlm.nih.gov/genbank/'><b>Genbank</b></a></font> | ||
| + | |||
| + | <br><br><br> | ||
| + | |||
| + | |||
| + | <font size = 3><b>2. Do any of the new BioBrick parts (or devices) that you made this year raise safety issues?</b></font size><br><br> | ||
| + | |||
| + | |||
| + | <font size = 3><b><center>Potential BioBrick safety concerns:</center></b></font size><br> | ||
| + | We are not aware of any dangers with any of the biobrick parts that we are using in the project. We will continue to monitor for any issues with the parts as the project progresses.</font size><br><br><br> | ||
| + | |||
| + | <font size = 3><b>3. Is there a local biosafety group, committee, or review board at your institution?</font size></b><br><br> | ||
| + | |||
| + | <font size = 2>Macquarie University Ryde, NSW Australia does have a Biosafety Committee; information on the committee can be accessed by the links below</font><br><br> | ||
| + | |||
| + | <font size = 2><center> | ||
| + | <a class="three" href='http://www.research.mq.edu.au/for/researchers/how_to_obtain_ethics_approval/biosafety_research_ethics'><b>Macquarie University - Bioethics approval</b></a><br> | ||
| + | <a class="three" href='http://web.science.mq.edu.au/intranet/ohs/hazsub/biosafety.htm#Biosafety'><b>Macquarie University - Biosafety</b> </a></center></font> | ||
| + | <br><br> | ||
| + | |||
| + | |||
| + | <font size = 2>The committee complies with the Australian Government regulations and strongly urges researchers to be familiar with the Gene Technology Regulation Act (2000) and the Gene Technology Regulations (2001) as well as the Office of the Gene Technology Regulator (OGTR) which hold regulations and requirements to keep notifiable risks to a minimum. Experiments involving cloning or transformation of organisms must pass approval by the Biosafety committee in which we were granted (approval number REF: 5201001087EX) for the expression of genes involved in chlorophyll biosynthesis in-vitro <i>Escherichia coli</i>. </font><br><br><br> | ||
| + | |||
| + | |||
| + | <font size = 3><b>4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?</font size></b> | ||
| - | + | <br><br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | As we are modifying genetic material, we have considered control options and switches if the modified organism were to be exposed to the environment or used industrially. Whilst considering these factors are important, the shipping and receiving of biological items, such as BioBricks is also a key factor that needs to be understood in order to understand the regulations and specificity required based on a country and world wide as a market. Using this, future teams can devise control and safety protocols. | |
| - | + | <br><br> | |
| + | Samples must pass quarantine checks in many countries around the world. The various quarantine measures enforced by each country varies heavily, this results in not only highly varied shipping and receiving times, but also fees for processing potential biological materials. So we have decided to aid in increasing awareness of possible quarantine measures performed within countries which have competing iGEM teams. | ||
| - | < | + | <a class="three" href='https://2013.igem.org/Team:Macquarie_Australia/Quarantine'><b>Quarantine Link</b></a> |
| - | + | ||
| - | + | <br><br><br> | |
| - | + | </html> | |
| - | + | ||
| - | <font size = | + | <font size = 5> Submitted Safety Forms </font> |
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | < | + | [[File:Safety1MQ1.png|140px|thumb|left|Our safety forms - Part 1 (<i>Click to Enlarge</i>)]][[File:Safety2MQ1.png|140px|thumb|left|Our safety forms - Part 2 (<i>Click to Enlarge</i>)]] |
| - | < | + | [[File:Safety3MQ1.png|140px|thumb|left|Our safety forms - Part 3 (<i>Click to Enlarge</i>)]] |
| - | + | [[File:Safety4MQ1.png|140px|thumb|left|Our safety forms - Part 4 (<i>Click to Enlarge</i>)]] | |
| + | [[File:Safety5MQ1.png|140px|thumb|left|Our safety forms - Part 5 (<i>Click to Enlarge</i>)]] | ||
Latest revision as of 17:27, 27 September 2013





iGEM Safety Questions
Project Safety concerns
To minimise risk of contamination or harm to researchers participating in the study or the environment, all students involved in the iGEM Macquarie University 2013 team received safety information according to the Macquarie University’s safety guidelines. The team also experienced an induction on how to operate specific machines within the lab, for example how to safely use the electroporator when dealing with chemicals and containers. We were supervised at all times by a senior member, or professor when working within the labs. A few examples of safe practices we employed to avoid biological material contamination include; disposal of biological waste in biohazard bins, correct labelling of materials, disinfect (with ethanol) the benches both before and after use and adhere to safe laboratory practices (including wearing lab coats, glasses and enclosed shoes etc.). Also to protect personnel and the environment, the chemicals involved in the lab work were used according to MSDS standards.
The host cells we used were Escherichia coli Star, which is categorised as a Biosafety Level 1 according to the World Health Organisation (WHO). Thus this E. coli strain has a low health risk to humans, the community and the environment. The plasmid pSB1S3 was provided by iGEM and is identified as a low safety risk (BSL1). The gBlocks, vector DNA and chlorophyll genes (which originated form Chalymodmonas reinhardtii) and were meticulously handled according to the gene regulations of the Australian Government’s Office of the Gene Technology Regulator, whom abide by the Gene Technology Act 2000.
Pathogenic sequences? Search Genbank
2. Do any of the new BioBrick parts (or devices) that you made this year raise safety issues?
We are not aware of any dangers with any of the biobrick parts that we are using in the project. We will continue to monitor for any issues with the parts as the project progresses.
3. Is there a local biosafety group, committee, or review board at your institution?
Macquarie University Ryde, NSW Australia does have a Biosafety Committee; information on the committee can be accessed by the links below
Macquarie University - Biosafety
The committee complies with the Australian Government regulations and strongly urges researchers to be familiar with the Gene Technology Regulation Act (2000) and the Gene Technology Regulations (2001) as well as the Office of the Gene Technology Regulator (OGTR) which hold regulations and requirements to keep notifiable risks to a minimum. Experiments involving cloning or transformation of organisms must pass approval by the Biosafety committee in which we were granted (approval number REF: 5201001087EX) for the expression of genes involved in chlorophyll biosynthesis in-vitro Escherichia coli.
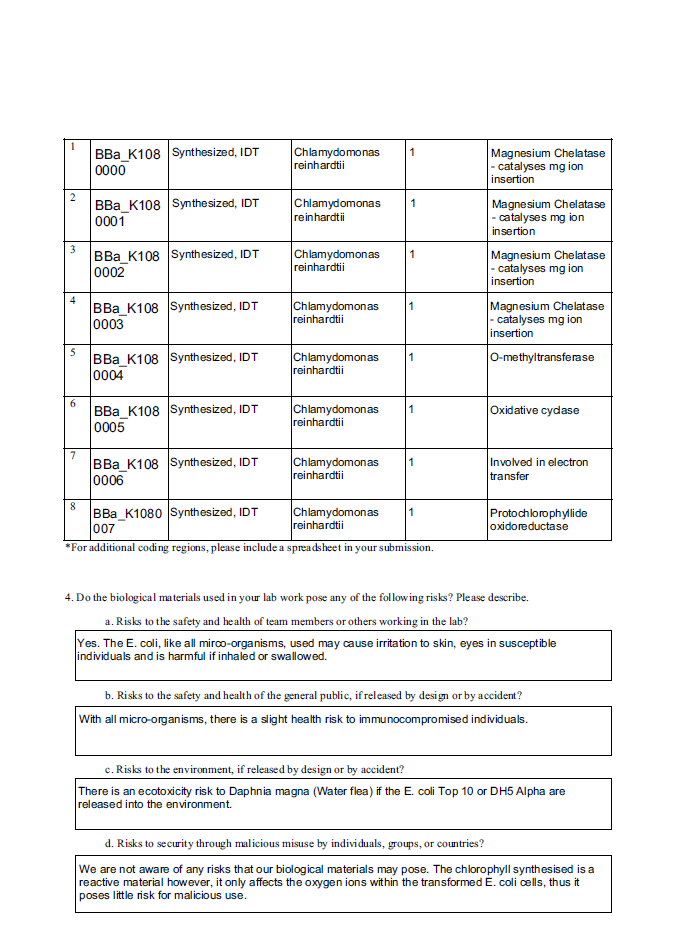
4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
As we are modifying genetic material, we have considered control options and switches if the modified organism were to be exposed to the environment or used industrially. Whilst considering these factors are important, the shipping and receiving of biological items, such as BioBricks is also a key factor that needs to be understood in order to understand the regulations and specificity required based on a country and world wide as a market. Using this, future teams can devise control and safety protocols.
Samples must pass quarantine checks in many countries around the world. The various quarantine measures enforced by each country varies heavily, this results in not only highly varied shipping and receiving times, but also fees for processing potential biological materials. So we have decided to aid in increasing awareness of possible quarantine measures performed within countries which have competing iGEM teams. Quarantine Link
Submitted Safety Forms
 "
"