Template:Kyoto/Notebook/Sep 5
From 2013.igem.org
(Difference between revisions)
(→Colony PCR) |
(→Colony PCR) |
||
| Line 508: | Line 508: | ||
|5min||30s||30s||1min 25s||30cycles | |5min||30s||30s||1min 25s||30cycles | ||
|} | |} | ||
| - | [[ | + | [[FileIgku Sep6 ColonyPCRN1.jpg]] |
</div> | </div> | ||
Revision as of 19:23, 25 September 2013
Sep 5
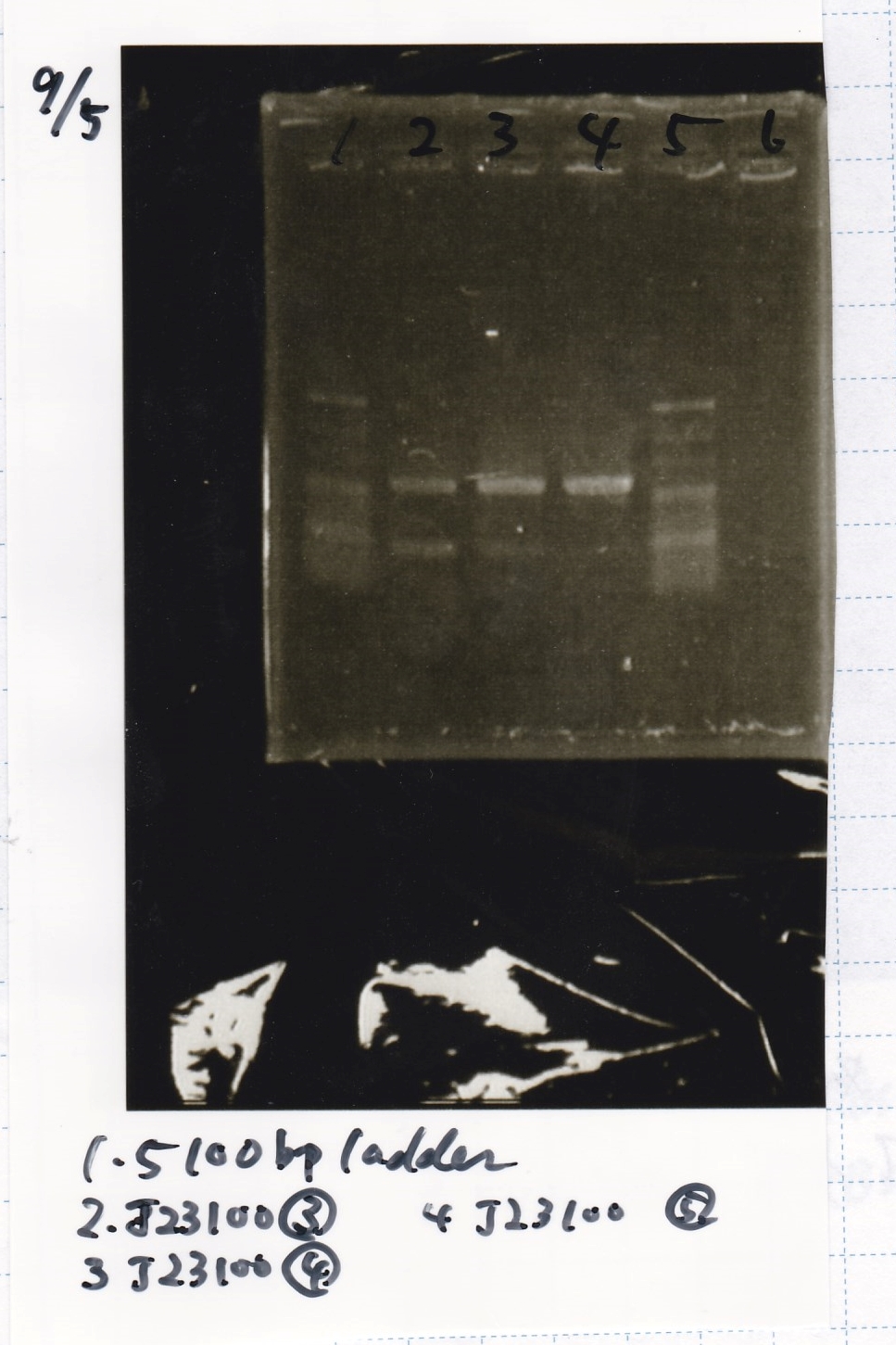
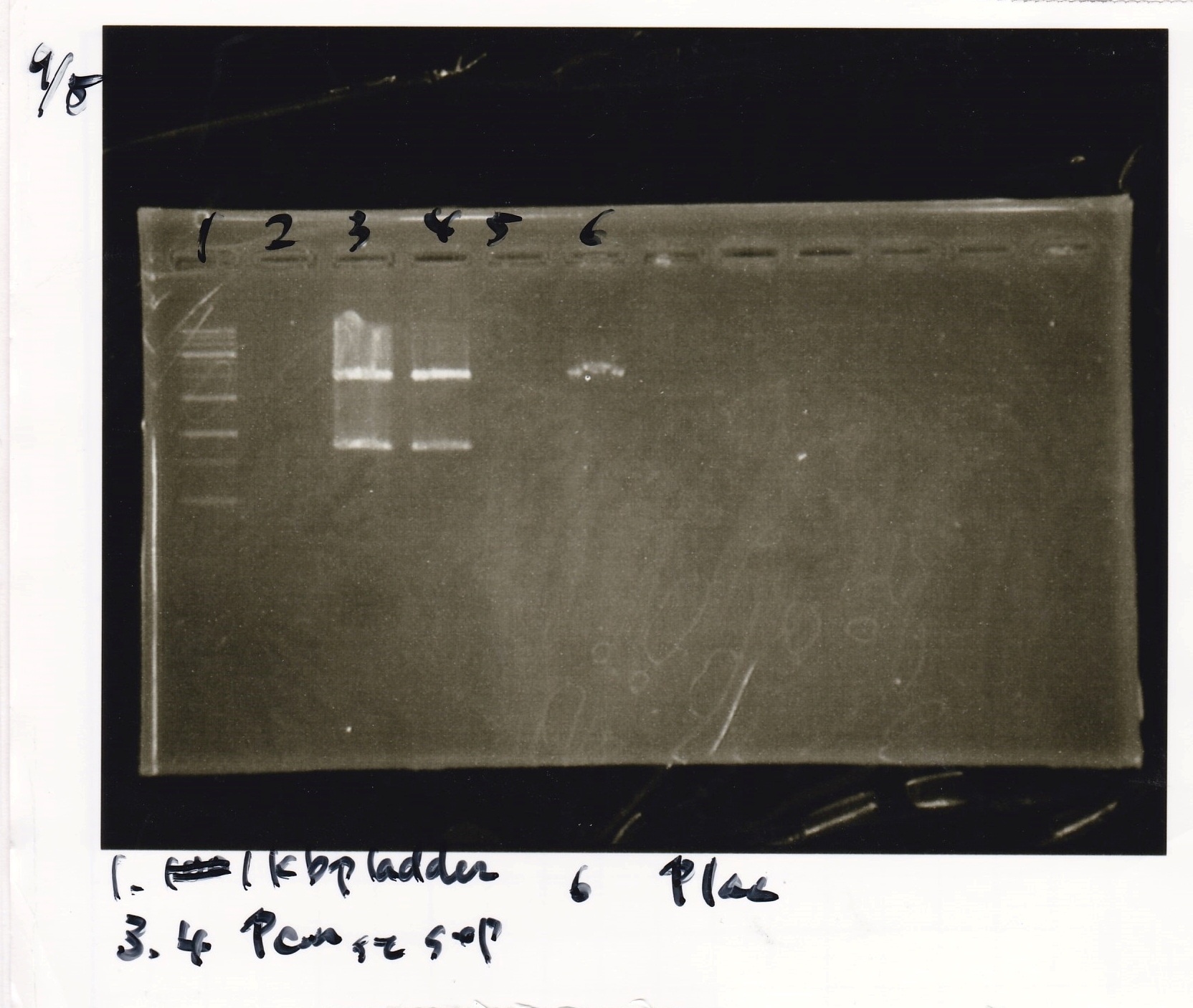
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | J23100-3 | -- | -- |
| 3 | J23100-4 | -- | -- |
| 4 | J23100-5 | -- | -- |
| 5 | 100bp ladder | -- | -- |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/3 J23100-3 | 349.1 | 1.69 | 1.851 |
| 8/10 Plac-2 | 331.3 | 1.84 | 1.42 |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | Pconst | 13.2 | spinach-DT | 2.1 | 3.5 |
| experiment | Plac | 15.3 | spinach-DT | 2.1 | 3.5 |
| experiment | Pconst | 13.2 | pT181 antisense (XbaI & PstI) | 6.6 | 3.5 |
| experiment | Plac | 15.3 | pT181 antisense (XbaI & PstI) | 6.6 | 3.5 |
| experiment | Ptet | 30.1 | pT181 antisense (XbaI & PstI) | 6.6 | 3.5 |
| experiment | Pconst | 13.2 | pT181 attenuator | 11.7 | 3.5 |
| experiment | Plac | 15.3 | pT181 attenuator | 11.7 | 3.5 |
| experiment | Ptet | 30.1 | RBS-lacZα-DT | 3.0 | 3.5 |
incubate 16 °C 1 hour
Electrophoresis
Gel Extraction
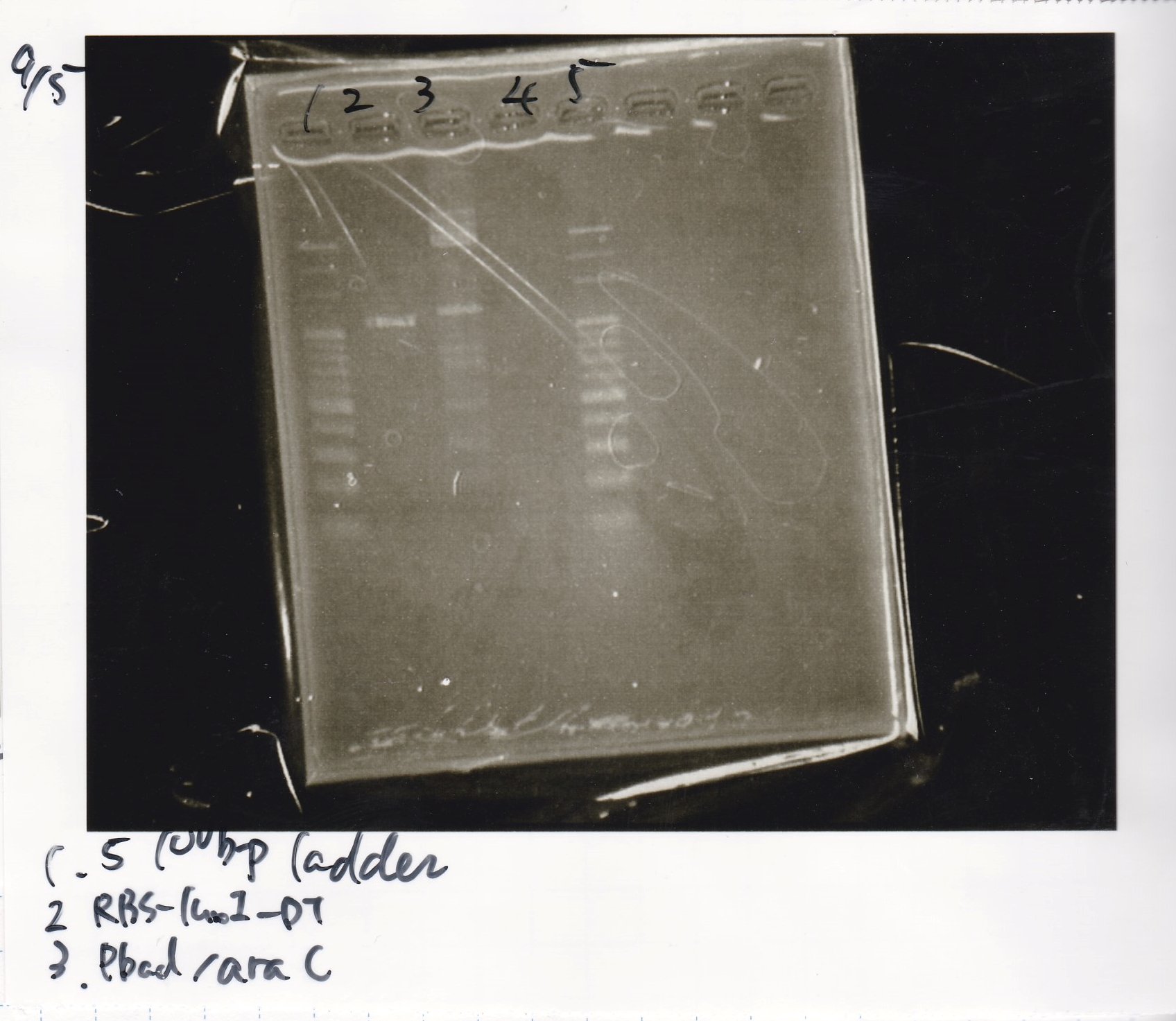
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | RBS-luxI-DT | |
| 3 | RBS-luxI-DT | |
| 4 | ||
| 5 | Pbad/araC | |
| 6 | Pbad/araC |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| RBS-luxI-DT | 23.2 | 0.83 | 7.89 |
| Pbad/araC | 51.8 | 1.20 | 0.11 |
Colony PCR
| Sample | base pair |
|---|---|
| 9/4 aptamer 12_1R(pSB1C3)-1 | 384 |
| 9/4 aptamer 12_1R(pSB1C3)-2 | 384 |
| 9/4 pT181 antisense(pSB1C3)-1 | 415 |
| 9/4 pT181 antisense(pSB1C3)-2 | 415 |
| 9/4 pT181 antisense(pSB1C3)-3 | 415 |
| 9/4 pT181 antisense(pSB1C3)-4 | 415 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 30s | 30cycles |
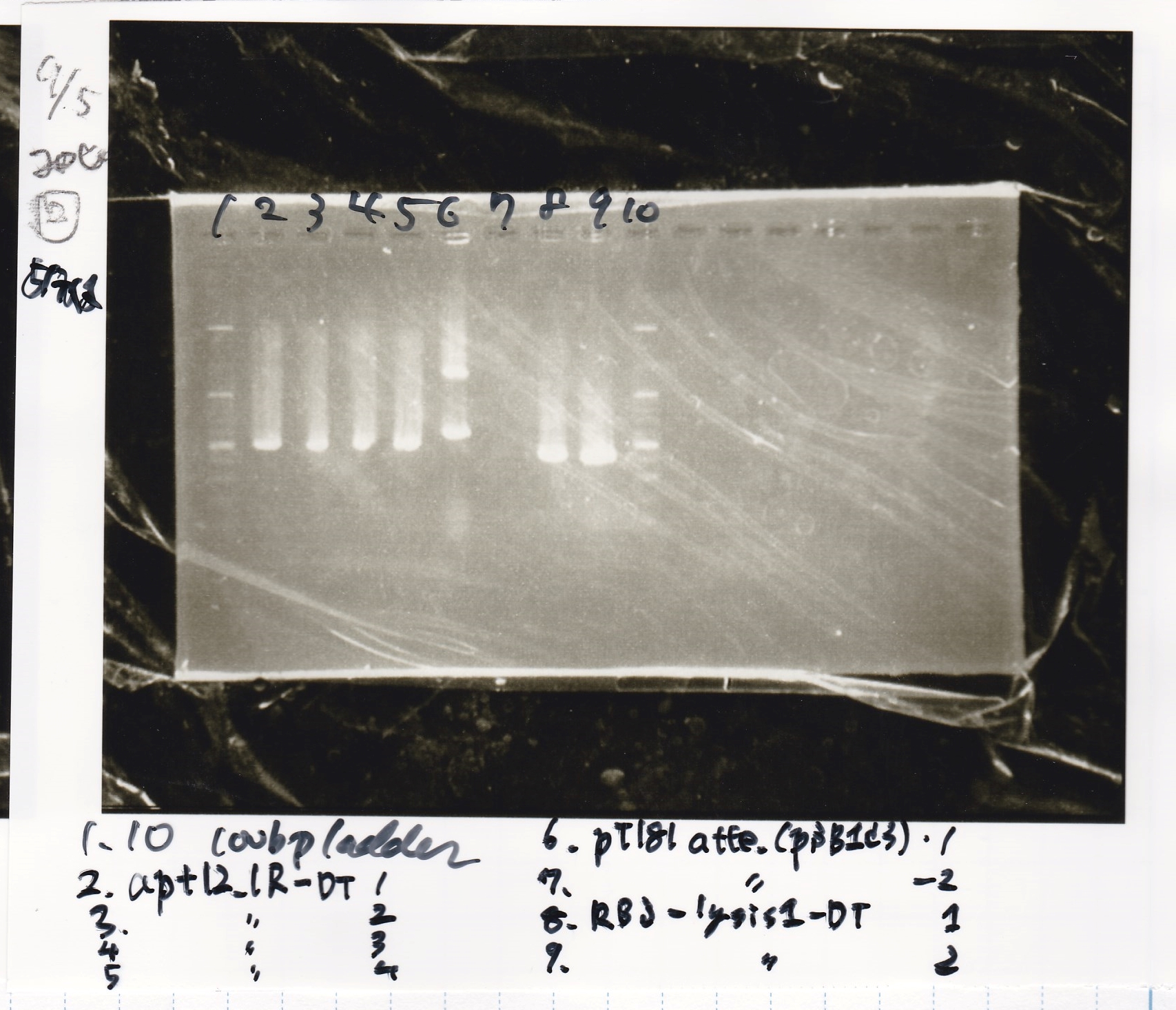
| Sample | base pair |
|---|---|
| 9/4 aptamer 12_1R-DT-1 | 521 |
| 9/4 aptamer 12_1R-DT-2 | 521 |
| 9/4 aptamer 12_1R-DT-3 | 521 |
| 9/4 aptamer 12_1R-DT-4 | 521 |
| 9/4 pT181 attenuator(pSB1C3) | 601 |
| 9/4 pT181 attenuator(pSB1C3) | 601 |
| 9/4 RBS-lysis1-DT | 613 |
| 9/4 RBS-lysis1-DT | 613 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 36s | 30cycles |
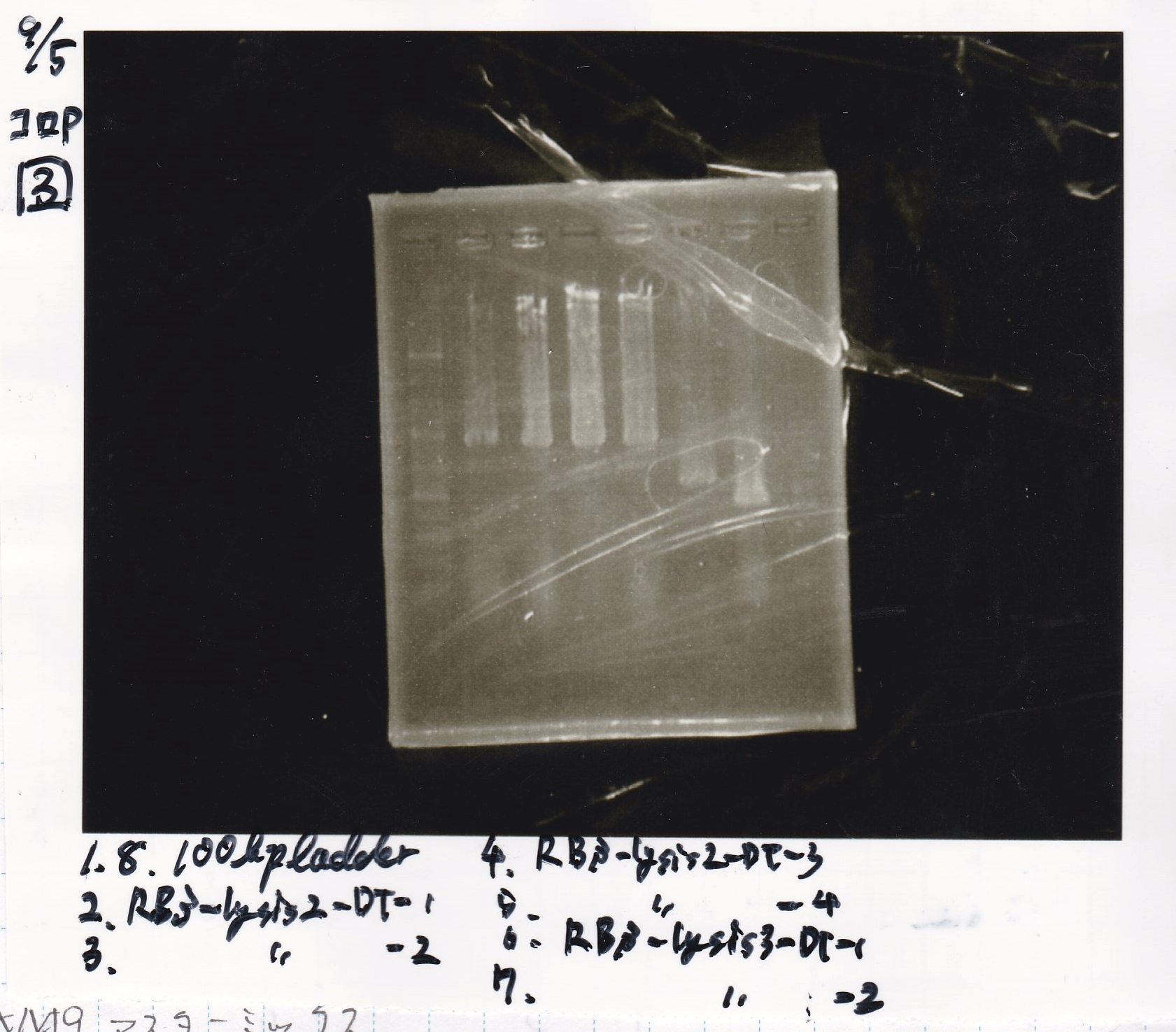
| Sample | base pair |
|---|---|
| 9/4 RBS-lysis2-DT-1 | 985 |
| 9/4 RBS-lysis2-DT-2 | 985 |
| 9/4 RBS-lysis2-DT-3 | 985 |
| 9/4 RBS-lysis2-DT-4 | 985 |
| 9/4 RBS-lysis3-DT-1 | 1210 |
| 9/4 RBS-lysis3-DT-2 | 1210 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 72s | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| 8/9 J23100-5 | Plusgrow medium(+Amp) |
37°C
Transformation
| Name | Sample(µL) | Competent Cells(µL) | Total(µL) | Plate |
|---|---|---|---|---|
| 9/5 Pconst+spinach-DT | 2 | 20 | 22 | -- |
| 9/5 Plac+spinach-DT | 2 | 20 | 22 | -- |
| 9/5 Pconst+pT181 antisense | 2 | 20 | 22 | -- |
| 9/5 Plac+pT181 antisense | 2 | 20 | 22 | -- |
| 9/5 Ptet+pT181 antisense | 2 | 20 | 22 | -- |
| 9/5 Plux+pT181 attenuator | 2 | 20 | 22 | -- |
| 9/5 Ptet+pT181 attenuator | 2 | 20 | 22 | -- |
| 9/5 Ptet+pT181 attenuator | 2 | 20 | 22 | -- |
| 9/5 Pconst+RBS-tetR+DT | 2 | 20 | 22 | -- |
| 9/16(2012) T7-His-FT | 2 | 20 | 22 | -- |
| 9/16(2012) pBr322 | 2 | 20 | 22 | -- |
Restriction Enzyme Digestion
| 9/5 Pbad/araC | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 4µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 18µL | 30µL |
| 9/5 RBS-luxI-DT | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 4µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 18µL | 30µL |
| 8/28 Plux | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 12µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 10µL | 30µL |
| NC | 0.6µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.4µL | 10µL |
| 9/5 Pconst(J23100) | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 5.7µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 16.3µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 9/5 Plac | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 6.0µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 16µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL |
| 8/31 Plux-RBS-GFP-DT-1 | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 9.4µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 12.6µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
| 8/20 Pconst-RBS-luxR-DT | E | S | X | P | BSA | Buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 4.5µL | 1µL | 0µL | 1µL | 0µL | 3µL | 3µL | 13.5µL | 30µL |
| NC | 0.2µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.8µL | 10µL |
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/5 RBS-luxI-DT | 7.6 | 1.56 | -1.42 |
| 9/5 Pbad/araC | 7.5 | 1.54 | 3.84 |
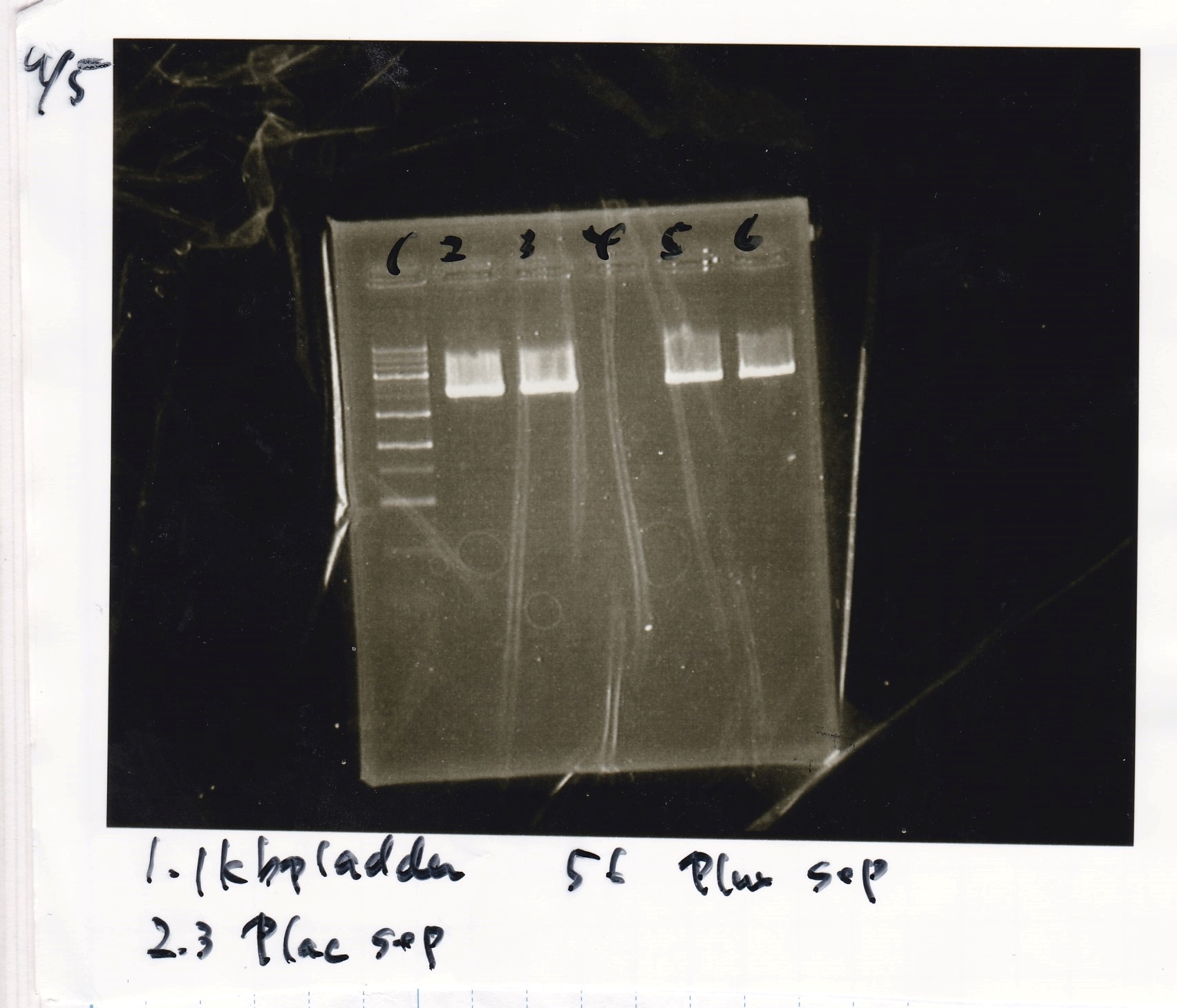
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | aptamer 12_1R (pSB1C3)-1 | -- | -- |
| 3 | aptamer 12_1R (pSB1C3)-2 | -- | -- |
| 4 | pT181 antisense(pSB1C3)-1 | -- | -- |
| 5 | pT181 antisense(pSB1C3)-2 | -- | -- |
| 6 | pT181 antisense(pSB1C3)-3 | -- | -- |
| 7 | pT181 antisense(pSB1C3)-4 | -- | -- |
| 8 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | aptamer 12_1R-DT-1 | -- | -- |
| 3 | aptamer 12_1R-DT-2 | -- | -- |
| 4 | aptamer 12_1R-DT-3 | -- | -- |
| 5 | aptamer 12_1R-DT-4 | -- | -- |
| 6 | pT181 attenuator(pSB1C3)-1 | -- | -- |
| 7 | pT181 attenuator(pSB1C3)-2 | -- | -- |
| 8 | RBS-lysis1-DT-1 | -- | -- |
| 9 | RBS-lysis1-DT-2 | -- | -- |
| 10 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 100bp ladder | -- | -- |
| 2 | RBS-lysis2-DT-1 | -- | -- |
| 3 | RBS-lysis2-DT-2 | -- | -- |
| 4 | RBS-lysis2-DT-3 | -- | -- |
| 5 | RBS-lysis2-DT-4 | -- | -- |
| 6 | RBS-lysis3-DT-1 | -- | -- |
| 7 | RBS-lysis3-DT-2 | -- | -- |
| 8 | 100bp ladder | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kbp ladder | -- | -- |
| 2 | Pconst | SpeI | PstI |
| 3 | Pconst NC | -- | -- |
| 4 | Plac | SpeI | PstI |
| 5 | Plac NC | -- | -- |
| 6 | Plux | SpeI | PstI |
| 7 | Plux NC | -- | -- |
| 8 | Pconst-RBS-luxR-DT | EcoRI | XbaI |
| 9 | Pconst-RBS-luxR-DT NC | -- | -- |
| 10 | Plux-RBS-GFP-DT | EcoRI | SpeI |
| 11 | Plux-RBS-GFP-DT NC | -- | -- |
| 12 | 1kbp ladder | -- | -- |
5x M9 Medium (+EDTA)
| volume | 10ml |
|---|---|
| Na2HPO4 | 60mg |
| KH2PO4 | 30mg |
| NaCl | 5mg |
| NH4Cl | 10mg |
| Fe(III)-EDTA | 1263.27mg |
| MilliQ | up to 10 mL |
- autoclave at 121 °C for 20 min
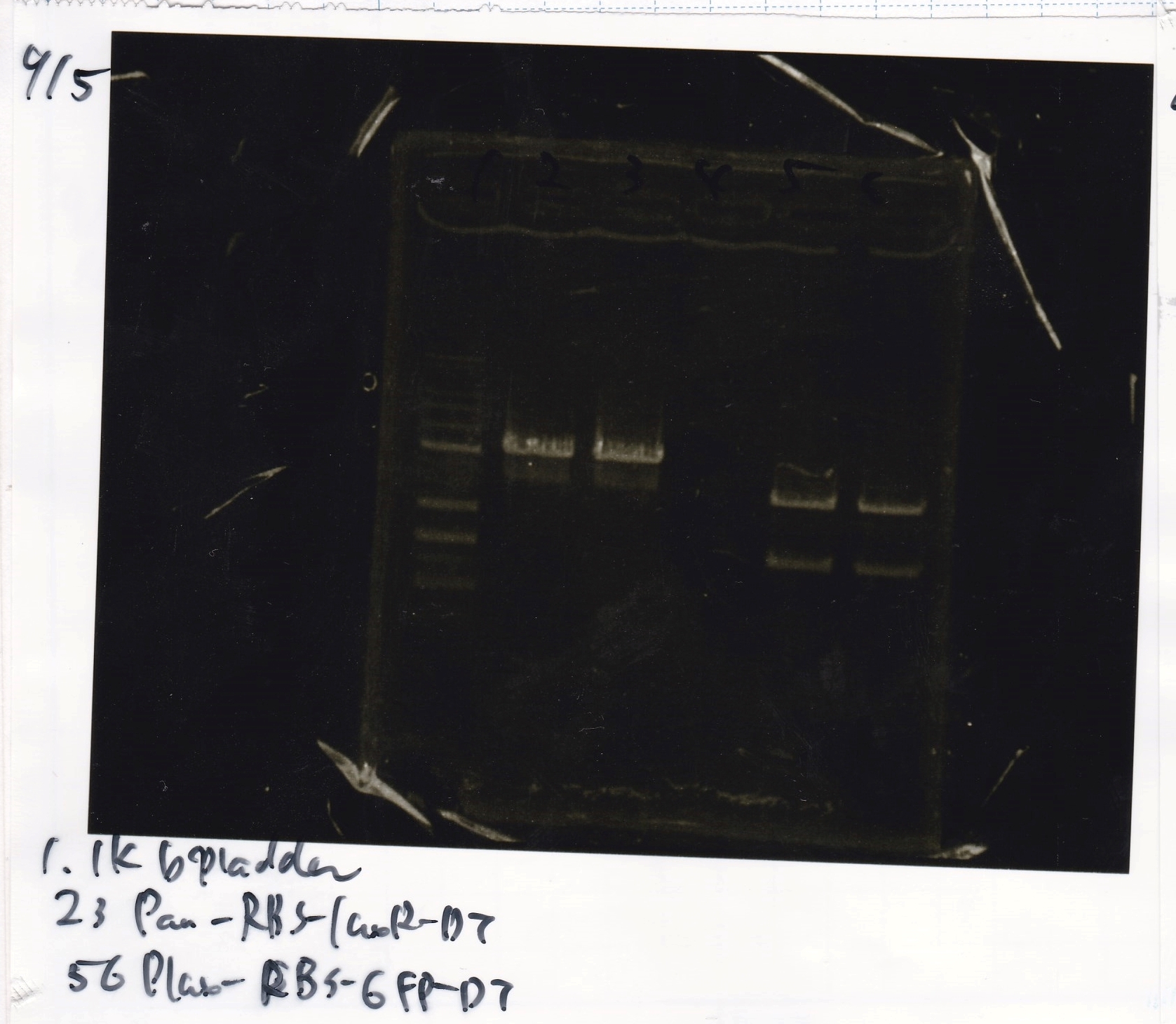
Gel Extraction
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | Pconst | SpeI & PstI |
| 3 | Pconst | SpeI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pconst | 5.3 | 1.98 | 0.06 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 100bp ladder | |
| 2 | Plac | SpeI & PstI |
| 3 | Plac | SpeI & PstI |
| 5 | Plux | SpeI & PstI |
| 6 | Plux | SpeI & PstI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Plac (SpeI & PstI) | 5.2 | 1.82 | 0.36 |
| Plux(SpeI & PstI) | 8.0 | 1.87 | 0.25 |
| Lane | DNA | Enzyme |
|---|---|---|
| 1 | 1kbp ladder | |
| 2 | Pconst-RBS-luxR-DT | EcoRI & XbaI |
| 3 | Pconst-RBS-luxR-DT | EcoRI & XbaI |
| 5 | Plux-RBS-GFP-DT | EcoRI & SpeI |
| 6 | Plux-RBS-GFP-DT | EcoRI & SpeI |
| Name | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| Pconst-RBS-luxR-DT(EcoRI & XbaI) | 30.6 | 1.84 | 1.16 |
| Plux-RBS-GFP-DT (EcoRI & SpeI) | 21.8 | 1.87 | 0.98 |
DNA Purification
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/5 RBS-luxI-DT | 7.6 | 1.56 | -1.42 |
| 9/5 Pbad/araC | 7.5 | 1.54 | 3.84 |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 8/9 J23100-5 | 243.0 | 1.90 | 1.81 |
Colony PCR
| Sample | base pair |
|---|---|
| 9/4 RBS-lysis3-DT-3 | 1210 |
| 9/4 RBS-lysis3-DT-4 | 1210 |
| 9/4 RBS-lysis3-DT-5 | 1210 |
| 9/4 RBS-lysis1-DT-3 | 613 |
| 9/4 Ptrc KaiC-1 | -- |
| 9/4 pSB4K5 | 1370 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min 25s | 30cycles |
Liquid Culture
| Sample | medium |
|---|---|
| 9/4 aptamer 12_1R-DT-1 | Plusgrow medium (+CP) |
| 9/4 pT181 attenuator(pSB1C3)-1 | Plusgrow medium (+CP) |
| 9/4 RBS-lysis2-DT-1 | Plusgrow medium (+CP) |
| 9/4 Ptrc-KaiC -1 | Plusgrow medium (+Amp) |
| 9/4 pSB4K5 -1 | Plusgrow medium (+Kan) |
 "
"