Team:Carnegie Mellon/Project/Results
From 2013.igem.org
(Difference between revisions)
| Line 2: | Line 2: | ||
<br><br><br> | <br><br><br> | ||
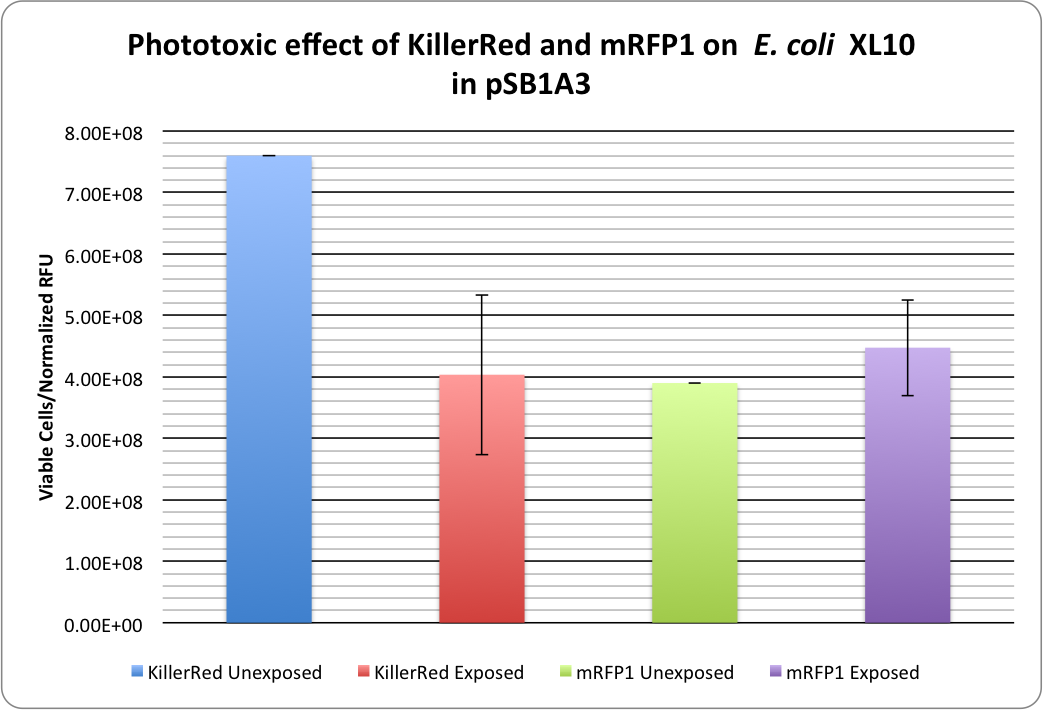
[[image:KillerRed RFP Phototoxicity1.png|thumb|600px|center|<b>Figure 1:</b> Phototoxicity of KillerRed and mRFP1 (<partinfo>BBa_E1010</partinfo>) on <i>E. coli</i> XL10 by irradiating with a HBO100 lamp (includes 375 nm LP filter) for 5 hours.]] | [[image:KillerRed RFP Phototoxicity1.png|thumb|600px|center|<b>Figure 1:</b> Phototoxicity of KillerRed and mRFP1 (<partinfo>BBa_E1010</partinfo>) on <i>E. coli</i> XL10 by irradiating with a HBO100 lamp (includes 375 nm LP filter) for 5 hours.]] | ||
| + | <br><br> | ||
<p>KillerRed's phototoxic effect on <i>E. coli</i> XL10 is shown in Figure 1.<br /> | <p>KillerRed's phototoxic effect on <i>E. coli</i> XL10 is shown in Figure 1.<br /> | ||
RFP 114% ± 20% (viable cells)/(Normalized RFU) <br /> | RFP 114% ± 20% (viable cells)/(Normalized RFU) <br /> | ||
KillerRed: 53% ± 17% (viable cells)/(Normalized RFU) | KillerRed: 53% ± 17% (viable cells)/(Normalized RFU) | ||
| - | </p> | + | </p><br><br> |
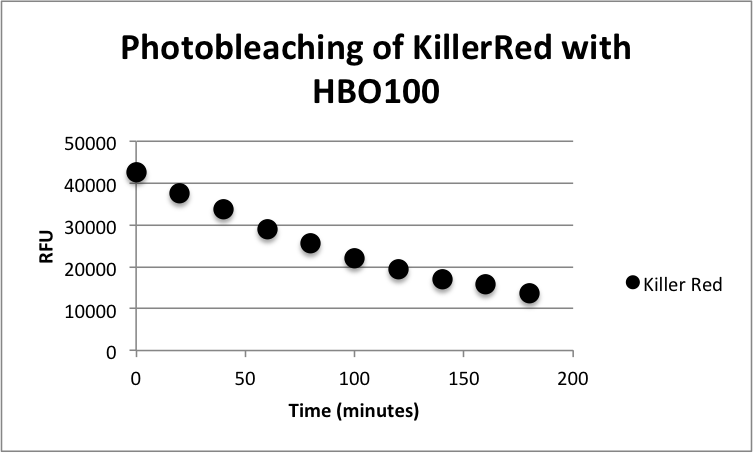
[[image:KR-Photobleach.png|thumb|600px|center|<b>Figure 1:</b> Photobleaching curve of KillerRed with a HBO100 mercury-arc lamp]] | [[image:KR-Photobleach.png|thumb|600px|center|<b>Figure 1:</b> Photobleaching curve of KillerRed with a HBO100 mercury-arc lamp]] | ||
<p>XL10 Ultracompetent cells were transformed with KillerRed (BBa_K1184000) cloned with <partinfo>BBa_B0034</partinfo> as the RBS and <partinfo>BBa_R0010</partinfo> as the wild-type lac promoter and induced overnight with IPTG.The overnight was bleached for 180 minutes with HBO100 (100W Mercury-arc lamp). Fluorescence data was taken using a Tecan Safire II with the parameters shown in Table 1. Fluorescence Data is shown in Table 2.</p> | <p>XL10 Ultracompetent cells were transformed with KillerRed (BBa_K1184000) cloned with <partinfo>BBa_B0034</partinfo> as the RBS and <partinfo>BBa_R0010</partinfo> as the wild-type lac promoter and induced overnight with IPTG.The overnight was bleached for 180 minutes with HBO100 (100W Mercury-arc lamp). Fluorescence data was taken using a Tecan Safire II with the parameters shown in Table 1. Fluorescence Data is shown in Table 2.</p> | ||
| - | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="40%"> | + | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="40%"> |
| + | <br><br> | ||
<caption><p align="justify"><b>Table 1</b> Tecan Safire<sup>2</sup> Parameters</p></caption> | <caption><p align="justify"><b>Table 1</b> Tecan Safire<sup>2</sup> Parameters</p></caption> | ||
<tr><td><b>Excitation (nm)</b></td><td>585</td></tr> | <tr><td><b>Excitation (nm)</b></td><td>585</td></tr> | ||
| Line 17: | Line 19: | ||
<tr><td><b>Number of reads</b></td><td>10</td></tr> | <tr><td><b>Number of reads</b></td><td>10</td></tr> | ||
<tr><td><b>Integration Time (microseconds)</b></td><td>40</td></tr> | <tr><td><b>Integration Time (microseconds)</b></td><td>40</td></tr> | ||
| + | <br><br> | ||
</table> | </table> | ||
<table cellpadding="2" border="1px" cellspacing="0" align="center" width="40%"> | <table cellpadding="2" border="1px" cellspacing="0" align="center" width="40%"> | ||
| Line 32: | Line 35: | ||
<tr><td align="left">180</td><td align="right">13741</td></tr> | <tr><td align="left">180</td><td align="right">13741</td></tr> | ||
</table> | </table> | ||
| + | <br><br> | ||
Revision as of 05:14, 27 September 2013
KillerRed's phototoxic effect on E. coli XL10 is shown in Figure 1.
RFP 114% ± 20% (viable cells)/(Normalized RFU)
KillerRed: 53% ± 17% (viable cells)/(Normalized RFU)
XL10 Ultracompetent cells were transformed with KillerRed (BBa_K1184000) cloned with <partinfo>BBa_B0034</partinfo> as the RBS and <partinfo>BBa_R0010</partinfo> as the wild-type lac promoter and induced overnight with IPTG.The overnight was bleached for 180 minutes with HBO100 (100W Mercury-arc lamp). Fluorescence data was taken using a Tecan Safire II with the parameters shown in Table 1. Fluorescence Data is shown in Table 2.
| Excitation (nm) | 585 |
| Emission (nm) | 610 |
| Excitation bandwidth (nm) | 10 |
| Emission bandwidth (nm) | 10 |
| Gain | 129 |
| Number of reads | 10 |
| Integration Time (microseconds) | 40 |
| Time (minutes) | Fluorescence (RFU) |
| 0 | 42598 |
| 20 | 37616 |
| 40 | 33749 |
| 60 | 29059 |
| 80 | 25680 |
| 100 | 21985 |
| 120 | 19442 |
| 140 | 17031 |
| 160 | 15738 |
| 180 | 13741 |
 "
"