Template:Kyoto/Notebook/Sep 26
From 2013.igem.org
(Difference between revisions)
(→Colony PCR) |
(→PCR) |
||
| Line 366: | Line 366: | ||
</div> | </div> | ||
| - | === | + | ===Ligasion(Golden Gate Assenbly)=== |
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !state||Vector||Inserter1||Inserter2||Inserter3||Inserter4 | ||
| + | |- | ||
| + | |experiment||GGA2||Pcon-attenuator(E-1A)||Pcon-tetR-DT(1-2A)||Pcon-pT181 attenuator(1-SA)| | ||
| + | |- | ||
| + | |experiment||GGA3||Pcon-tetap-DT(E-1A)||Pcon-tetR-DT(1-2A)||Pcon-GFP-DT(1-SA)||1.5 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||GGA4||Pcon-spi-DT(E-1A)||Pcon-tetR-DT(1-2A)||tet aptamer(1-3)||0.3 µL||Pcon-tetR-DT(3-2A)||1.1 µL||Ptet(2-5)| | ||
| + | |- | ||
| + | |experiment||GGA5||Pcon-antisense(E-1A)||Pcon-pT181 attenuator-DT(0-1A)||1.0 µL||Pcon-tetR-DT(1-2A)||1.0 µL||Ptet(2-5)||0.3µL||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||GGA6||Pcon-attenuator(E-1A)||1.0 µL||Pcon antisense-spinach-DT(1-2A)||0.7µL||Pcon-pT 181 attenuator(2-SA)||2.1 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||GGA7||Pcon-tetR-DT(E-2A)||0.5 µL||spinach-DT(1-2)||1.0 µL||Pcon-GFP-DT(1-2)||0.8 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |} | ||
| + | </div> | ||
Revision as of 07:07, 27 September 2013
Contents |
Sep 26
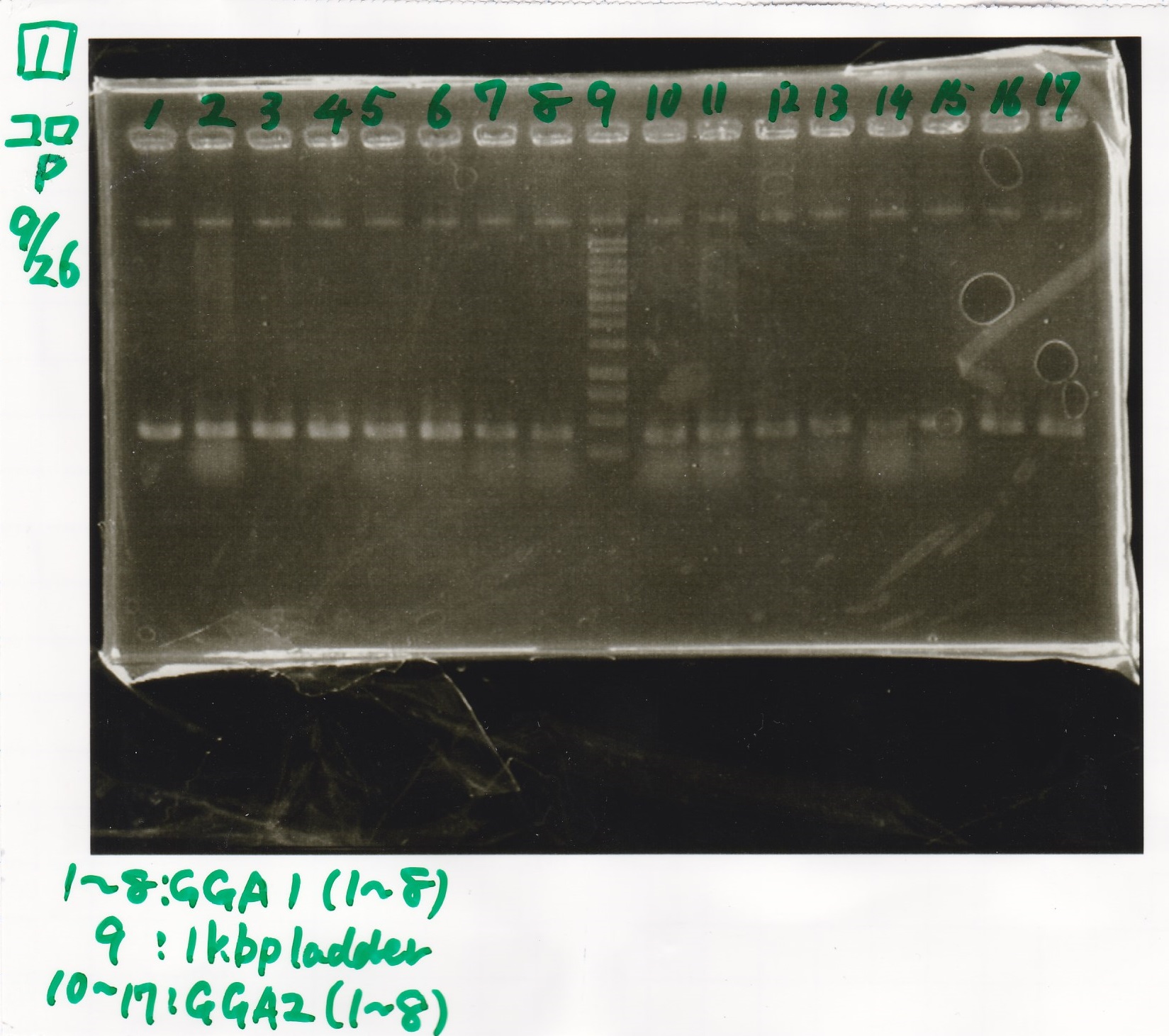
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA1-1 | ||
| 2 | GGA1-2 | ||
| 3 | GGA1-3 | ||
| 4 | GGA1-4 | ||
| 5 | GGA1-5 | ||
| 6 | GGA1-6 | ||
| 7 | GGA1-7 | ||
| 8 | GGA1-8 | ||
| 9 | 1kbp ladder | ||
| 10 | GGA2-1 | ||
| 11 | GGA2-2 | ||
| 12 | GGA2-3 | ||
| 13 | GGA2-4 | ||
| 14 | GGA2-5 | ||
| 15 | GGA2-6 | ||
| 16 | GGA2-7 | ||
| 17 | GGA2-8 |
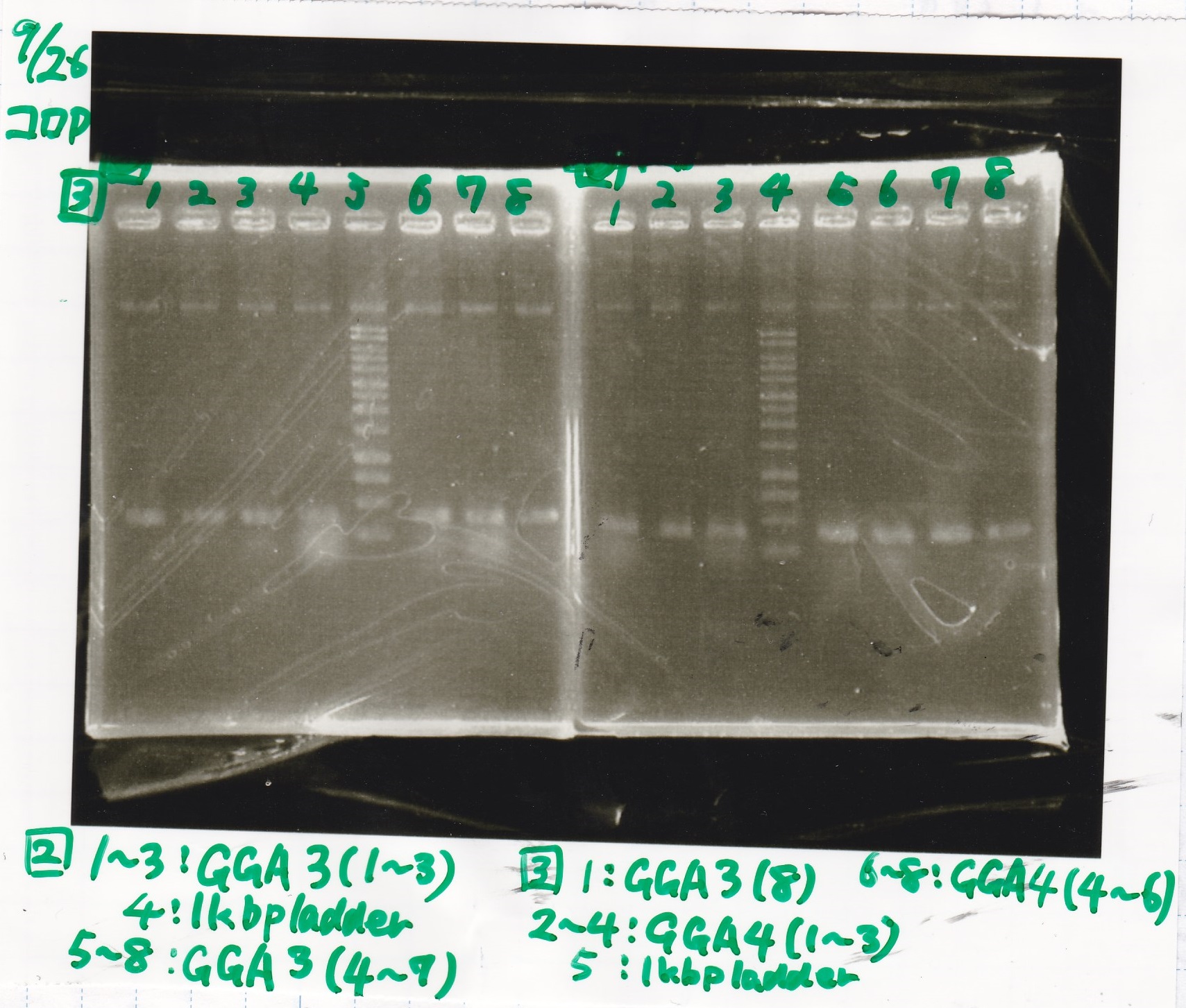
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA3-1 | ||
| 2 | GGA3-2 | ||
| 3 | GGA3-3 | ||
| 4 | 1kbp ladder | ||
| 5 | GGA3-4 | ||
| 6 | GGA3-5 | ||
| 7 | GGA3-6 | ||
| 8 | GGA3-7 |
</div>
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA3-8 | ||
| 2 | GGA4-1 | ||
| 3 | GGA4-2 | ||
| 4 | GGA4-3 | ||
| 5 | 1kbp ladder | ||
| 6 | GGA4-4 | ||
| 7 | GGA4-5 | ||
| 8 | GGA4-6 |
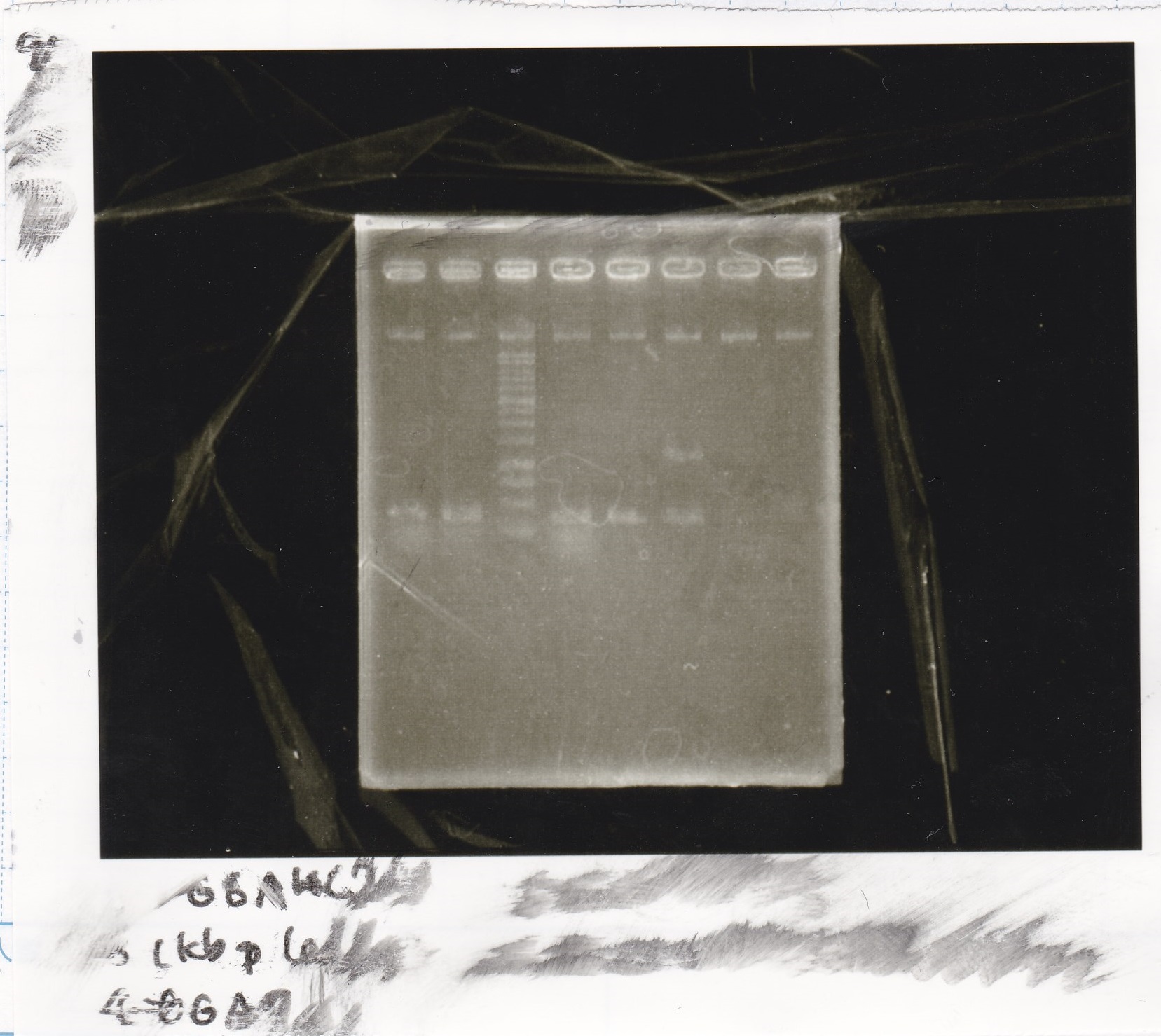
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA4-7 | ||
| 2 | GGA4-8 | ||
| 3 | 1kbp ladder | ||
| 4 | GGA7-1 | ||
| 5 | GGA16-1 | ||
| 6 | NC(RBS-GFP-DT) |
Ligasion(Golden Gate Assenbly)
| state | Vector | Inserter1 | Inserter2 | Inserter3 | Inserter4 | Inserter5 | (NEB)T4 ligase | (NEB)10*T4 ligase | MilliQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| experiment | RBS-GFP-DT | 1.0 µL | Pcon-pT181 antisense(E-2A) | 0.6µL | DT(2-1) | 0.5µL | Pcon-pT181 attenuator(1-SA) | 2.1 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | pSB1C3(EcoRI&SpeI) | 1.8 µL | Pcon-pT181 antisense(E-2A) | 0.9 µL | DT(2-1) | 0.5 µL | Pcon-GFP-DT(1-SA) | 1.5 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Ptet(E-0) | 0.7µL | Pcon pT181 attenuator(0-1A) | 0.3µL | tet aptamer(1-3) | 0.3 µL | Pcon-tetR-DT(3-2A) | 1.1 µL | Ptet(2-5) | 0.3 µL | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Ptet(E-0) | 0.7 µL | Pcon-pT181 attenuator-DT(0-1A) | 1.0 µL | Pcon-tetR-DT(1-2A) | 1.0 µL | Ptet(2-5) | 0.3µL | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Pcon-pT181 attenuator-tetR-DT(E-1A) | 1.0 µL | Pcon antisense-spinach-DT(1-2A) | 0.7µL | Pcon-pT 181 attenuator(2-SA) | 2.1 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Pcon antisense(E-1A) | 0.5 µL | spinach-DT(1-2) | 1.0 µL | Pcon-GFP-DT(1-2) | 0.8 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | pSB1C3(EcoRI&SpeI) | 1.8 µL | Pcon-pT181 antisense(E-1A) | 0.8µL | spinach-DT(1-2) | 1.0µL | Pcon-GFP-DT(2-SA) | 0.8 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | pSB1C3(EcoRI&SpeI) | 1.8 µL | Pcon-tet aptamer-DT(E-1A) | 3.0 µL | Ptet-pT181 antisense(1-3) | 1.5 µL | spinach-DT(3-2) | 1.5 µL | Pcon-GFP-DT(2-5A) | 0.8 µL | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-GFP-DT | Amp |
| RBS-GFP-DT | CP |
| Pcon-pT181 attenuator-DT | Amp |
| Pcon-tet aptamer-DT | Amp |
| Pcon-spinach-DT | Amp |
| Pcon-pT181 antisense-spinach-DT | Amp |
| spinach-DT | CP |
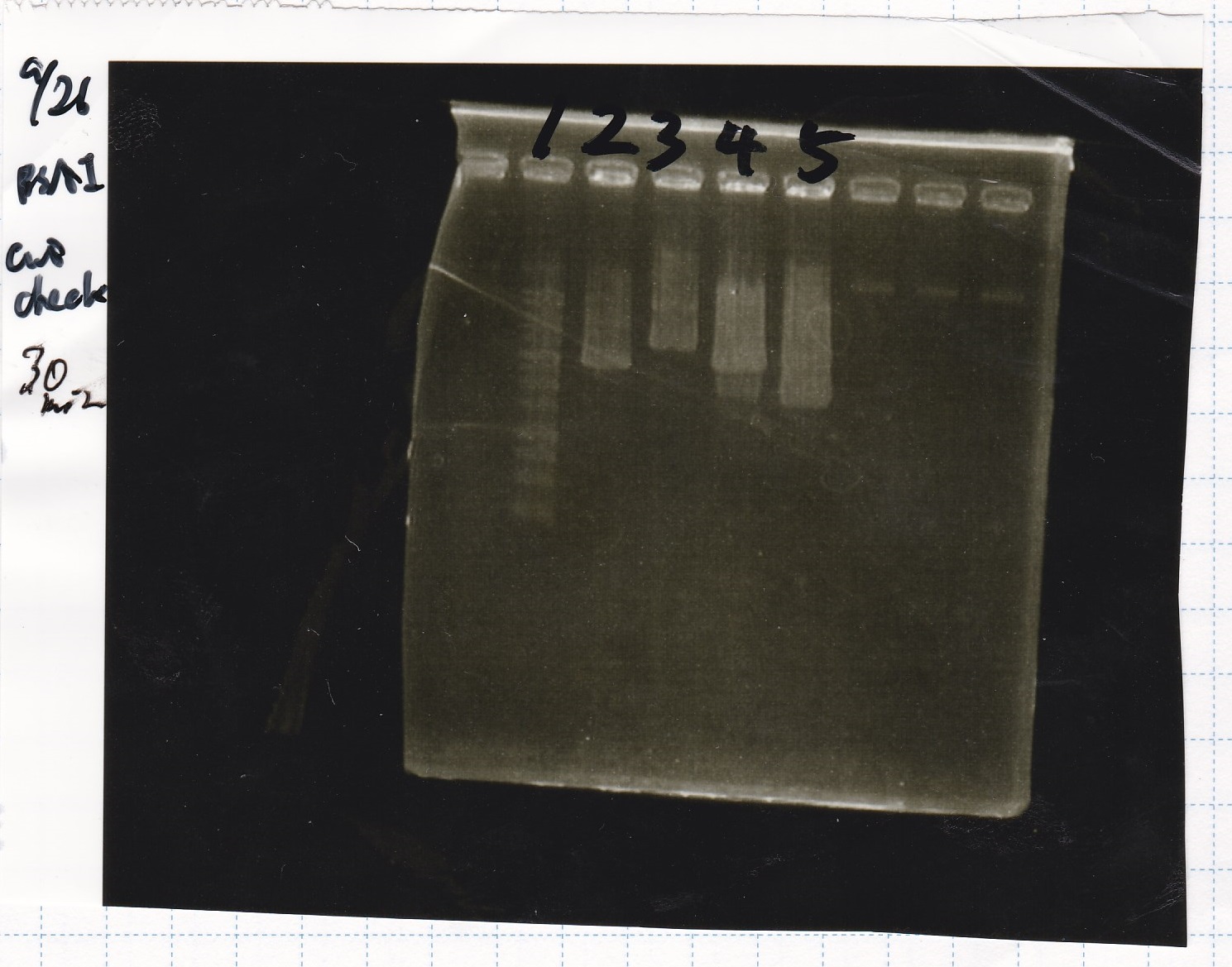
BSAI Digestion
| DNA | MilliQ | BSAI-HF | 10*cut smart | total | |
|---|---|---|---|---|---|
| Pcon | 7 µL | 10.7 µL | 0.3 µL | 2 µL | 20 µL |
| RBS-luxR-DT | 10 µL | 7 µL | 0.3 µL | 2 µL | 20 µL |
Colony PCR
| Sample | base pair |
|---|---|
| GGA-1 (9~16) | 3177 |
| GGA-2 (9~16) | 3189 |
| GGA-3 (9~21) | 2989 |
| GGA-4 (9~16) | 2939 |
| GGA-6 (1) | 3359 |
| GGA-16(2~4) | 2939 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 3min30s | 30cycles |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-1 (9) | -- | -- |
| 2 | GGA-1 (10) | -- | -- |
| 3 | GGA-1 (11) | -- | -- |
| 4 | GGA-1 (12) | -- | -- |
| 5 | GGA-1 (13) | -- | -- |
| 6 | GGA-1 (14) | -- | -- |
| 7 | GGA-1 (15) | -- | -- |
| 8 | GGA-1 (16) | -- | -- |
| 9 | 1kbp ladder | -- | -- |
| 10 | GGA-2 (9) | -- | -- |
| 11 | GGA-2 (10) | -- | -- |
| 12 | GGA-2 (11) | -- | -- |
| 13 | GGA-2 (12) | -- | -- |
| 14 | GGA-2 (13) | -- | -- |
| 15 | GGA-2 (14) | -- | -- |
| 16 | GGA-2 (15) | -- | -- |
| 17 | GGA-2 (16) | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-4 (9) | -- | -- |
| 2 | GGA-4 (10) | -- | -- |
| 3 | GGA-4 (11) | -- | -- |
| 4 | GGA-4 (12) | -- | -- |
| 5 | GGA-4 (13) | -- | -- |
| 6 | GGA-4 (14) | -- | -- |
| 7 | GGA-4 (15) | -- | -- |
| 8 | GGA-4 (16) | -- | -- |
| 9 | 1kbp ladder | -- | -- |
| 10 | GGA-6 (1) | -- | -- |
| 11 | GGA-16 (2) | -- | -- |
| 12 | GGA-16 (3) | -- | -- |
| 13 | GGA-16 (4) | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-3 (9) | -- | -- |
| 2 | GGA-3 (10) | -- | -- |
| 3 | GGA-3 (11) | -- | -- |
| 4 | GGA-3 (12) | -- | -- |
| 5 | 1kbp ladder | -- | -- |
| 6 | GGA-3 (13) | -- | -- |
| 7 | GGA-3 (15) | -- | -- |
| 8 | GGA-3 (14) | -- | -- |
</div>
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-3 (17) | -- | -- |
| 2 | GGA-3 (18) | -- | -- |
| 3 | GGA-3 (19) | -- | -- |
| 4 | 1kbp ladder | -- | -- |
| 5 | GGA-3 (20) | -- | -- |
| 6 | GGA-3 (21) | -- | -- |
| 7 | GGA-3 (16) | -- | -- |
Electrophoresis
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-GFP-DT | -- |
| RBS-GFP-DT | -- |
| Pcon-tet aptamer-DT | -- |
| Pcon-spinach-DT | -- |
| Pcon-pT181 antisense-spinach-DT | -- |
| spinach-DT | -- |
Colony PCR
| Sample | base pair |
|---|---|
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-1 | -- |
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-2 | -- |
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-3 | -- |
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-4 | -- |
| 9/24 Ptet-RBS-GFP-DT-1 | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min40s | 30cycles |
Ligasion(Golden Gate Assenbly)
| state | Vector | Inserter1 | Inserter2 | Inserter3 | Inserter4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| experiment | GGA2 | Pcon-attenuator(E-1A) | Pcon-tetR-DT(1-2A) | |||||||||||
| experiment | GGA3 | Pcon-tetap-DT(E-1A) | Pcon-tetR-DT(1-2A) | Pcon-GFP-DT(1-SA) | 1.5 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL | ||
| experiment | GGA4 | Pcon-spi-DT(E-1A) | Pcon-tetR-DT(1-2A) | tet aptamer(1-3) | 0.3 µL | Pcon-tetR-DT(3-2A) | 1.1 µL | |||||||
| experiment | GGA5 | Pcon-antisense(E-1A) | Pcon-pT181 attenuator-DT(0-1A) | 1.0 µL | Pcon-tetR-DT(1-2A) | 1.0 µL | Ptet(2-5) | 0.3µL | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL | |
| experiment | GGA6 | Pcon-attenuator(E-1A) | 1.0 µL | Pcon antisense-spinach-DT(1-2A) | 0.7µL | Pcon-pT 181 attenuator(2-SA) | 2.1 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | GGA7 | Pcon-tetR-DT(E-2A) | 0.5 µL | spinach-DT(1-2) | 1.0 µL | Pcon-GFP-DT(1-2) | 0.8 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
 "
"