From 2013.igem.org
(Difference between revisions)
|
|
| Line 144: |
Line 144: |
| | |} | | |} |
| | </div> | | </div> |
| - | <Ul>
| + | *=be evaporated |
| - | <Li Type="square">*=be evaporated</Li>
| + | |
| - | <Ul>
| + | |
| | | | |
| | ===Transformation=== | | ===Transformation=== |
Revision as of 20:09, 27 September 2013
Sep 9
PCR
Nakagawa
| KaiA(plasmid) | 10x buffer | dNTPs | MgSO4 | Primer(661-RBS-KaiA-fwd) | Primer(kaiA-662-rev-ver2) | Kod-plus ver2 | MilliQ | total
|
| 1.0 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 58.5°C | 68°C | --
|
| 2min | 10s | 30s | 30s | 30cycles
|
| KaiB(plasmid) | 10x buffer | dNTPs | MgSO4 | Primer(661-RBS-KaiA-fwd-ver2) | Primer(kaiB-663-rev-ver2) | Kod-plus ver2 | MilliQ | total
|
| 1.0 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 58.5°C | 68°C | --
|
| 2min | 10s | 30s | 30s | 30cycles
|
| P-KaiBC(PCR products) | 10x buffer | dNTPs | MgSO4 | Primer(prefix-P-kaiBC-fwd-ver2) | Primer(P-KaiBC-suffix-rev-verw) | Kod-plus ver2 | MilliQ | total
|
| 0.7 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.8 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 58.5°C | 68°C | --
|
| 2min | 10s | 30s | 30s | 30cycles
|
| DT(plasmid) | 10x buffer | dNTPs | MgSO4 | Primer(664-DT-fwd-ver2) | Primer(DT-suffix-660-rev-ver2) | Kod-plus ver2 | MilliQ | total
|
| 1.0 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 0.5 | 15.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 58.5°C | 68°C | --
|
| 2min | 10s | 30s | 30s | 30cycles
|
Yoshida
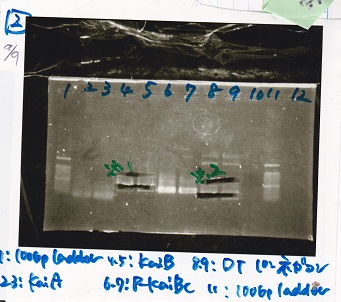
| Lane | DNA | Enzyme
|
| 1 | 100bp ladder | --
|
| 2 | KaiA | --
|
| 3
|
| 4 | KaiB | --
|
| 5
|
| 6 | P-KaiBC | --
|
| 7
|
| 8 | DT | --
|
| 9
|
| 10 | NC | --
|
| 11 | 100bp ladder | --
|

| Name | concentration[µg/mL] | 260/280 | 260/230
|
| KaiC | 28.5 | 1.26 | 11.92
|
| Plac | 53.9 | 1.82 | 1.61
|
| KaiB | 59.6 | 1.61 | 2.20
|
| KaiB | 76.5 | 1.86 | 1.44
|
| DT | 98.7 | 1.47 | 1.43
|
Miniprep
No name
| DNA | concentration[µg/mL] | 260/280 | 260/230
|
| 9/8 pSB1C3 | 83.2 | 1.45 | 0.76
|
| 9/8 RBS-lysis2-DT | 72.8 | 1.67 | 0.97
|
| 9/8 spinach-DT | 152.6 | 1.88 | 1.97
|
| Nuclease-Free water | -0.4 | -14.30 | 0.25
|
Restriction Enzyme Digestion
No name
| | 9/9 pSB1C3 | XbaI | PstI | Buffer | BSA | MilliQ | total
|
| 2 cuts | 24µL | 1µL | 1µL | 3µL | 3µL | 22µL | 30µL*
|
| NC | 1.2µL | 0µL | 0µL | 1µL | 1µL | 6.8 µL | 10µL
|
| | 9/9 pSB1C3 | XbaI | PstI | Buffer | BSA | MilliQ | total
|
| 2 cuts | 27.4µL | 1µL | 1µL | 3µL | 3µL | 22µL | 30µL*
|
| NC | 1.3µL | 0µL | 0µL | 1µL | 1µL | 6.7µL | 10µL
|
| | 9/9 spinach-DT | XbaI | PstI | Buffer | BSA | MilliQ | total
|
| 2 cuts | 10.8µL | 1µL | 1µL | 3µL | 3µL | 22µL | 30µL*
|
| NC | 0.5µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL
|
Transformation
No name
| Name | Sample | Competent Cells | Total | Plate
|
| Pcon+RBS-luxI-DT | 2µL | 20µL | 22µL | Amp
|
| Pcon-RBS-luxR-DT+Plux-GFP | 2µL | 20µL | 22µL |
|
| Ptet-PT181 antisense+spinach-DT-Pbad/araC+RBS-lux-DT | 2µL | 20µL | 22µL |
|
Ligation
No Name
| state | Vector | Inserter | Ligation High ver.2
|
| experiment | 9/8 F1-attenuator(XbaI&PstI) | 5.3µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg
|
| experiment | 9/8 F3m2-attenuator(XbaI&PstI) | 4.5µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg
|
| experiment | 9/8 F1-antisense(XbaI&PstI) | 1.7µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg
|
| experiment | 9/8 F6-antisense(XbaI&PstI) | 2.9µg | 9/8 pSB1C3(XbaI&PstI) | 8.9µg | 3.5µg
|
| experiment | 9/8 aptamer12-P(EcoRI&SpeI) | 3.1µg | 9/8 pSB1C3(EcoRI&SpeI) | 8.8µg | 3.5µg
|
| experiment | 9/8 aptamer12-1M(EcoRI&SpeI) | 3.1µg | 9/8 pSB1C(EcoRI&SpeI)3 | 8.8µg | 3.5µg
|
M9 medium
Shimizu & Hirano
| EDTA consenlation | Na2HPO4 | KH2PO4 | NaCl | NH4Cl | Fe(Ⅲ)-EDTA | distilled water | total
|
| 1mMol | 300mg | 150mg | 25mg | 50mg | 21mg | up to 10mL | 10mL
|
| 10mMol | 300mg | 150mg | 25mg | 50mg | 210.5mg | up to 10mL | 10mL
|
| 50mMol | 300mg | 150mg | 25mg | 50mg | 1052.7mg | up to 10mL | 10mL
|
Colony PCR
No name
| Sample | base pair
|
| 9/8 Plac-spinach-DT-(1) | 659
|
| 9/8 Plac-spinach-DT-(2) | 659
|
| 9/8 Pcon-pt181attenuator-DT-(1) | 584
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 94°C | 55°C | 68°C | --
|
| 5min | 30s | 30s | 40s | 30cycles
|
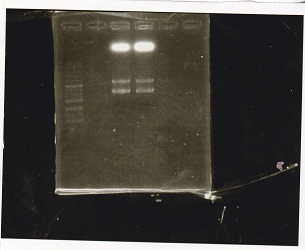
No name
| Lane | DNA | Enzyme
|
| 1 | 100bp ladder | --
|
| 2 | Spinach-DT | XbaI+PstI
|
| 3
|
| 5 | RBS-lacZα-DT | XbaI+PstI
|
| 6
|

| Lane | DNA | Enzyme
|
| 1 | 100bp ladder | --
|
| 3 | pSB1C3 | XbaI+PstI
|
| 4
|
Ligation
No name
| state | Vector | Inserter | Ligation High ver.2
|
| experiment | pSB1C3(XbaI+PstI) | xx µL | 9/8 F1-attenuator(XbaI+PstI) | xx µL | xx µL
|
| experiment | pSB1C3(XbaI+PstI) | xx µL | 9/8 F3m2-attenuator(XbaI+PstI) | xx µL | xx µL
|
PCR
Tatsui and Yoshida
| KaiC | 10x buffer KOD plus ver2 | 2M dNTP | MgSO4 | Primer Mix(F&R) | Kod-plus ver2 | MilliQ | total
|
| 0.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.0 | 25.0
|
| 1.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.5 | 25.0
|
| 2.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25.0
|
| 4.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 12.5 | 25.0
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 2min | 30cycles
|
| Plac(100pg) | 10x buffer KOD plus ver2 | 2M dNTP | MgSO4 | Primer Mix(F&R) | Kod-plus ver2 | MilliQ | total
|
| 0.3 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.2 | 25.0
|
| 0.6 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.9 | 25.0
|
| 1.2 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.3 | 25.0
|
| 2.4 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.1 | 25.0
|
| 0.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.5 | 25.0
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 2min | 30cycles
|
Liquid Culture
No name
| Sample | medium
|
| pSB2C3(master)-2 | Plusgrow medium(+CP)
|
Restriction Enzyme Digestion
No name
| | 8/17 lysis1-2 | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 8.1µL | 1µL | 1µL | 3µL | 3µL | 13.9µL | 30µL
|
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL
|
| | 8/17 lysis3-1 | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 6.1µL | 1µL | 1µL | 3µL | 3µL | 15.9µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 9/15 Plac | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 1 cuts | 12.1µL | 0µL | 1µL | 3µL | 3µL | 10.9µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
PCR
Yoshida
| KaiA(KaiABC) 100pg/µL | 10x buffer KOD plus ver2 | dNTPs | MgSO4 | Primer Mix(Fwd&Rev) | KOD plus ver2 | MilliQ | total
|
| 2.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25
|
| 4.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 12.5 | 25
|
| 8.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 8.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 60°C | 68°C | --
|
| 2min | 10s | 30s | 1min | 30cycles
|
| PKaiBC 400pg/µL | 10x buffer KOD plus ver2 | dNTPs | MgSO4 | Primer Mix(Fwd&Rev) | KOD plus ver2 | MilliQ | total
|
| 0.5 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 16.0 | 25
|
| 1.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 15.5 | 25
|
| 2.0 | 2.5 | 2.5 | 1.5 | 1.5 | 0.5 | 14.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 60°C | 68°C | --
|
| 2m | 10s | 30s | 1m | 30cycles
|
Liquid Culture
No name
| Sample | medium
|
| 9/2 Spinach-DT | Plusgrow medium(+CP)
|
| 8/29 Plux-RBS-GFP-DT | Plusgrow medium
|
| 9/6 master plate | Plusgrow medium(+Amp)
|
| 3 Pcon-PT181 attenuator | Plusgrow medium
|
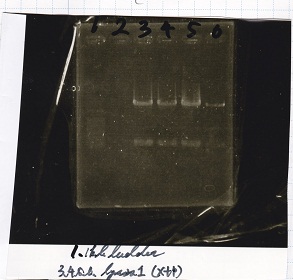
No name
| Lane | DNA | Enzyme
|
| 1 | 1kbp ladder | --
|
| 3 | lysis1 | XbaI+PstI
|
| 4
|
| 5
|
| 6
|

| Lane | DNA | Enzyme
|
| 1 | 1kbp ladder | --
|
| 3 | lysis3 | XbaI+PstI
|
| 4
|
| 5
|
| 6
|


| Name | concentration[µg/mL] | 260/280 | 260/230
|
| (´ω`) | | |
|
| (ΦωΦ^) | | |
|
Electrophoresis
Yoshida
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | 1kb ladder | -- | --
|
| 2 | Plac 100pg | -- | --
|
| 3 | Plac 200pg | -- | --
|
| 4 | Plac 400pg | -- | --
|
| 5 | Plac 800pg | -- | --
|
| 6 | NC | -- | --
|
| 7 | 1kbp ladder | -- | --
|

 "
"








