Team:Clemson/Notebook
From 2013.igem.org
(Difference between revisions)
| Line 4: | Line 4: | ||
{| | {| | ||
Our goal for this year’s project was to construct a universal self-amplifying biosensor (USAB) which could be used to quickly determine the presence or absence of any bacterial pathogen. The self-amplifying nature of our biosensor would allow for rapid and sensitive detection, and the universality of our biosensor would allow it to be adapted to detection of any bacterium. For more information on how our system would work, please see the Project page. | Our goal for this year’s project was to construct a universal self-amplifying biosensor (USAB) which could be used to quickly determine the presence or absence of any bacterial pathogen. The self-amplifying nature of our biosensor would allow for rapid and sensitive detection, and the universality of our biosensor would allow it to be adapted to detection of any bacterium. For more information on how our system would work, please see the Project page. | ||
| - | + | <br> | |
We wanted our system to respond to and amplify the quorum sensing AHL signals that would be sent from a pathogen (more details on this later), and respond by sending out a visible signal that we could measure or detect (e.g. GFP, chromogenic protein, etc.). An outline of our approach to synthesize and test our USAB is detailed below. | We wanted our system to respond to and amplify the quorum sensing AHL signals that would be sent from a pathogen (more details on this later), and respond by sending out a visible signal that we could measure or detect (e.g. GFP, chromogenic protein, etc.). An outline of our approach to synthesize and test our USAB is detailed below. | ||
| - | + | <br> | |
| - | + | <br> | |
'''Phase I: Construction of the Universal Self-Amplifying BioSensor (USAB)''' | '''Phase I: Construction of the Universal Self-Amplifying BioSensor (USAB)''' | ||
We actually decided to make three version of this; each would include the constitutively expressed activator luxR, the LuxR/AHL activated promoter PLux followed by the AHL-producing luxI and a signal output (GFP, amilCP, or mAAA). GFP would, of course, produce a fluorescent signal, amilCP is a blue chromogenic protein, or mAAA would enzymatically convert the colorless acetomenaphin to a reddish compound. The goal was to find out which of the three versions could inherently produce the most easily detectable signal output in the shortest amount of time. | We actually decided to make three version of this; each would include the constitutively expressed activator luxR, the LuxR/AHL activated promoter PLux followed by the AHL-producing luxI and a signal output (GFP, amilCP, or mAAA). GFP would, of course, produce a fluorescent signal, amilCP is a blue chromogenic protein, or mAAA would enzymatically convert the colorless acetomenaphin to a reddish compound. The goal was to find out which of the three versions could inherently produce the most easily detectable signal output in the shortest amount of time. | ||
<br> | <br> | ||
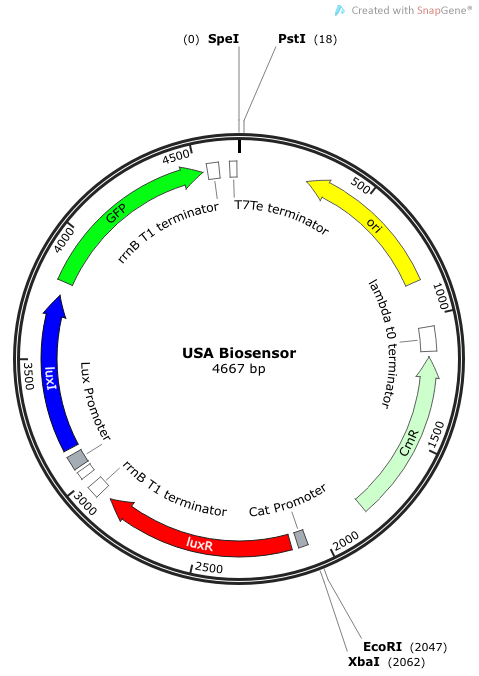
The plasmid construct for the USAB-GFP version is shown below. The other version would replace GFP with amilCP or mAAA. | The plasmid construct for the USAB-GFP version is shown below. The other version would replace GFP with amilCP or mAAA. | ||
| - | + | |- | |
|align="center"|[[File:USA Biosensor.png|175px|frameless|center]] | |align="center"|[[File:USA Biosensor.png|175px|frameless|center]] | ||
|[[File:USA Biosensor.png|175px|frameless|center]] | |[[File:USA Biosensor.png|175px|frameless|center]] | ||
| - | + | |- | |
''Rationale'': I14033 was used because the medium transcription rate would be less energetically burdensome on the cell, and this seemed reasonable for a transcriptional regulator. The purpose of having I0462, R0062, and C0261 was to create a positive feedback loop. I0462 would be activated by an outside source of AHLs thereby activating R0062. R0062 would then drive transcription of C0261 to express LuxI, which is responsible for synthesizing more AHLs to drive I0462. This positive feedback loop is what makes the sensor self-amplifying, thus making a very minute signal from a pathogen become more detectable in smaller concentrations. Detection is made possible by GFP or the colorimetric proteins amilCP and mAAA. | ''Rationale'': I14033 was used because the medium transcription rate would be less energetically burdensome on the cell, and this seemed reasonable for a transcriptional regulator. The purpose of having I0462, R0062, and C0261 was to create a positive feedback loop. I0462 would be activated by an outside source of AHLs thereby activating R0062. R0062 would then drive transcription of C0261 to express LuxI, which is responsible for synthesizing more AHLs to drive I0462. This positive feedback loop is what makes the sensor self-amplifying, thus making a very minute signal from a pathogen become more detectable in smaller concentrations. Detection is made possible by GFP or the colorimetric proteins amilCP and mAAA. | ||
<br> | <br> | ||
Revision as of 22:08, 27 September 2013
Notebook
 "
"