Team:Biwako Nagahama/Original 3A Assembly
From 2013.igem.org
(→“Protocol”) |
(→“Protocol”) |
||
| Line 281: | Line 281: | ||
<p>↓Mix</p> | <p>↓Mix</p> | ||
<p>37℃ 20min</p> | <p>37℃ 20min</p> | ||
| - | [[File:Biwako-Nagahama_E.P_syohei8.png|200px|]] | + | [[File:Biwako-Nagahama_E.P_syohei8.png|200px|right]] |
T7RNApolymerase-duble.terminater | T7RNApolymerase-duble.terminater | ||
Revision as of 22:51, 27 September 2013
3A Assembly Biwako-Nagahama original protocol
Reason
We succeeded in 3A Assembly using Linear Backbone from the Distribution Kit obtained from the iGEM Headquarter. But we could not succeeded in making 3A Assembly of the linear backbone taking reference of the Protocol of linear backbone found in the iGEM Homepage. So, we made our own 3A Assembly protocol suitable to our own lab environment that could increase the success rate of the experiment.
On discussing about the 3A Assembly with other participating teams, we found that other teams were not preparing their own protocol regarding 3A Assembly. We found that the 3A Assembly could be performed easily in the environment with certain restrictions. Because 3A Assembly is Assmbly method of the high probability not to need PCR and gel purification. So we tried to debug the available protocols and make our own protocols suitable to our own experimenting environment and help other teams with similar environment condition.
“Protocol”
<iGEM Backbone(pSB1C3,pSB1K3,pSB11A3,pSB1T3) manufacture>
2×KODFx buffer・・・25μL
dNTPs[2mM]・・・0μL
[http://parts.igem.org/Help:Protocols/Linearized_Plasmid_Backbones ※1] SB-prep-3P-1・・・1.5μL
[http://parts.igem.org/Help:Protocols/Linearized_Plasmid_Backbones ※2] SB-prep-2Ea・・・1.5μL
KODFx[1.0U/μL]・・・1.0μL
MilliQ H2O・・・10.0μL
Template[1~10ng/μL](pSB1C3 or pSB1K3 or pSB1T3 or pSB1A3)・・・1.0μL
Total・・・50.0μL
[http://www.toyobo-global.com/seihin/xr/lifescience/products/pcr_002.html ToYoBo KOD FX]<p/> <p>↓
94℃ 2min
↓
__________________
98℃ 10sec
58℃ 30sec 30cycles
68℃ 2min30sec
__________________
↓
10℃ ∞
↓
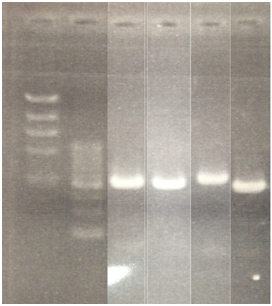
<Electrophoresis>
TE buffer・・・7μL
PCR Sample・・・2μL
10×Loading buffer・・・1μL
Total・・・10μL
0.7% Gel
________________________________
1 λ-HindⅢ 10μL
2 500bp DNA ladder 10μL
3 pSB1A3 10μL
4 pSB1K3 10μL
5 pSB1T3 10μL
6 pSB1C3 10μL
________________________________
↓
<Exo Star process>
ExoⅠ・・・0.1μL
BAP・・・0.1μL
MilliQ H2O・・・1.8μL
PCR Sample・・・17.0μL
Total・・・19.0μL
[http://193.218.17.133/ex/downloads/brochures/life_science/ge_illustra_exostar.pdf illustra™ ExoStar™ 1-Step]
↓
37℃ 30min
↓
80℃ 15min
↓
10℃ ∞
↓
<Phenol-chloroform extraction>
Add Phenol-chloroform where is equivalent to Exo Star process sample
↓Centrifuge 4℃ 13,000rpm 5min
Only supernatant was taken
↓
<EtOH precipitation>
Add 1μL 20mg/mL Glycogen
↓Mix
Add 1/10 volume 3M CH3COONa(pH5.2)
↓
Add 2.5 times volume 99.5% EtOH
↓Vortex
↓Centrifuge 4℃ 13,000rpm 20min
Waste supernatant
↓
Add 500μL 70% EtOH
↓Mix
↓Centrifuge for 10s in a table-top microcentrifuge
Waste supernatant
↓
65℃ Dry up
↓
Add 11μL TE buffer
↓
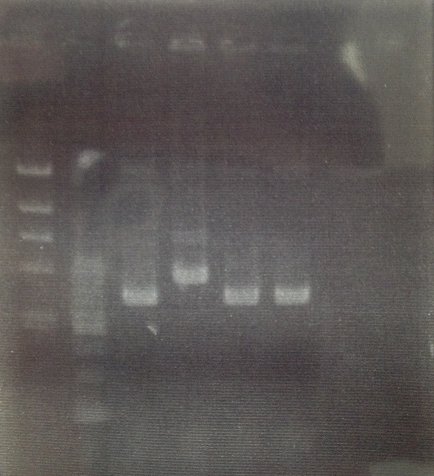
<Electrophoresis>
TE buffer・・・8μL
EtOH crystallization Sample・・・1μL
10×Loading buffer・・・1μL
Total・・・10μL
0.7% Gel
↓
<Restrictive Digestion>
Backbone template・・・500ng
dH2O・・・-50μL
10×H buffer・・・5μL
EcoRⅠ・・・1μL
PstⅠ・・・1μL
Total・・・50μL
↓
37℃ 1h
↓
<Phenol-chloroform extraction>
Add Phenol-chloroform where is equivalent to Exo Star process sample
↓Centrifuge 4℃ 13,000rpm 5min
Only supernatant was taken
↓
<EtOH precipitation>
Add 1μL 20mg/mL Glycogen<p> <p>↓Mix
Add 1/10 volume 3M CH3COONa(pH5.2)
↓
Add 2.5 times volume 99.5% EtOH
↓Vortex
↓Centrifuge 4℃ 13,000rpm 20min
Waste supernatant
↓
Add 500μL 70% EtOH
↓Mix
↓Centrifuge for 10s in a table-top microcentrifuge
Waste supernatant
↓
65℃ Dry up
↓
Add 10μL TE buffer
↓
<Restrictive Digestion>
EtOH precipitation Sample・・・10μL
dH2O・・・33μL
10×H buffer・・・5μL
EcoRⅠ・・・1μL
PstⅠ・・・1μL
Total・・・50μL
↓
37℃ Over Night
↓
<Phenol-chloroform extraction>
Add Phenol-chloroform where is equivalent to Exo Star process sample
↓Centrifuge 4℃ 13,000rpm 5min
Only supernatant was taken
↓
<EtOH precipitation>
Add 1μL 20mg/mL Glycogen
↓Mix
Add 1/10 volume 3M CH3COONa(pH5.2)
↓
Add 2.5 times volume 99.5% EtOH
↓Vortex
↓Centrifuge 4℃ 13,000rpm 20min
Waste supernatant
↓
Add 500μL 70% EtOH
↓Mix
↓Centrifuge for 10s in a table-top microcentrifuge
Waste supernatant
↓
65℃ Dry up
↓
Add 8μL TE buffer
↓
<Electrophoresis>
TE buffer・・・8μL
EtOH crystallization Sample・・・1μL
10×Loading buffer・・・1μL
Total・・・10μL
0.7% Gel
↓
<Ligation>
①insertA(20ng)
②insertB(20ng)
③Backbone template(20ng)
DNA Ligation Kit ver 2.1 ①+②+③μL
[http://www.clontech.com/takara/JP/Products/Molecular_Biology/Cloning_Mapping_and_cDNA_Synthesis/Quick_DNA_Ligation?sitex=10036:22372:US TaKaRa DNA Ligation Kit ver 2.1]
↓
16℃ 30min
↓
<Transformation>
Add all ligation solution into your desired 100μL of competent cells
↓
Hold on ice for 20min
↓
Heat shock at 42℃ for 30sec
↓quickly
On ice for 2min
↓
Add 900μL of SOCborth
↓
Hold at 37℃ for 30min
↓
Plating 100μL of DNA Transformation
↓Centrifuge 4℃ 13,000rpm 1min
Waste supernatant for 800μL, and pipetting on anther plate
↓
Plating all
↓
Incubate at 37℃ (over night)
↓
<Colony PCR>(check Trance formation)
Take a single colony from the plate and then mix it with 30μL of de-ionized water(dH2O)
↓
dH2O・・・12.7μL
10×NH4 buffer・・・2.5μL
50mM MgCl2・・・0.75μL
25mM dNTPs・・・2μL
10μM [http://parts.igem.org/Part:BBa_G1004 ※3] BBa_G1004・・・1μL
10μM [http://parts.igem.org/Part:BBa_G1005 ※4] BBa_G1005・・・1μL
BIO Taq(5U/μL)・・・0.05μL
Template(dH2O mixed colony)・・・5μL
Total・・・25μL
↓
95℃ 30sec
↓
95℃ 10sec
55℃ 20sec 30cycles
72℃ 2min
↓
72℃ 2min
↓
10℃ ∞
↓
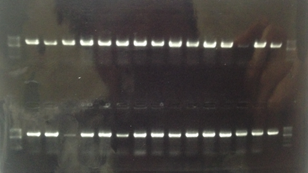
<Electrophoresis>
PCR Sample・・・9μL
10×Loading buffer・・・1μL
Total・・・10μL
0.7% Gel
_______________________________________________
1 500bp DNA ladder 10μL
2~16 Sample1~15 10μL
17 500bp DNA ladder 10μL
18 500bp DNA ladder 10μL
19~29 Sample16~30 10μL
30 500bp DNA ladder 10μL
_______________________________________________
Sample1~Sample30:T7RNApolymerase-Double Terminater on pSB1K3
<Miniprep>
1.5mL Culture fluid→New 1.5mL tube
↓Centrifuge 4℃ 13,000rpm 1min
Waste supernatant
↓
Add 200μl SolutionⅠ
↓vortex
Add 200μl SolutionⅡ
↓Mix(softy)
Add 200μl SolutionⅢ
↓Mix(softy)
Check to become cloudy
↓
On ice 5min
↓Centrifuge 4℃ 13,000rpm 10min
supernatant→New 1.5mL tube
↓
Add 500μL Isopropyl alchol
↓vortex
↓Centrifuge 4℃ 13,000rpm 20min
Waste supernatant
↓
Add 500μL 70% EtOH
↓Mix
↓Flash
Waste supernatant
↓
65℃ Dry up
↓
Add 50μL RNase in TE buffer
↓Mix
37℃ 20min
T7RNApolymerase-duble.terminater
By. Syohei Takeshita
 "
"