Team:Valencia Biocampus/Project
From 2013.igem.org
| Line 297: | Line 297: | ||
When we were considering setting up a transport of bacteria was thought to be necessary to have a 'destination', a place to go. That destiny would be a <b>'hot spot' on a heterogeneous substrate</b> where <b><i>Caenorhabditis</i> <i>elegans</i></b> should lead right to that point the bacteria. | When we were considering setting up a transport of bacteria was thought to be necessary to have a 'destination', a place to go. That destiny would be a <b>'hot spot' on a heterogeneous substrate</b> where <b><i>Caenorhabditis</i> <i>elegans</i></b> should lead right to that point the bacteria. | ||
<br/> | <br/> | ||
| - | Leveraging the powerful smell of the nematode, it was decided to try a number of attractants from various lists from | + | Leveraging the powerful smell of the nematode, it was decided to try a number of attractants from various lists from web <a href="http://www.wormbook.org">www.wormbook.org</a> that could work as 'hot spot' of our experiment. Thus, using the<i> C. elegans</i> <b>chemotaxis, </b>we could direct transport. |
</p> | </p> | ||
| Line 304: | Line 304: | ||
<h3>The attractants experiment</h3> | <h3>The attractants experiment</h3> | ||
| - | The test would be carried | + | The test would be carried creating our own plates on which half would be NGM unmodified and the other half part would be including soluble compounds before |
solidifying or after solidification in the case of volatiles. The list of modifications can be found in <b>Fig. 1</b>. | solidifying or after solidification in the case of volatiles. The list of modifications can be found in <b>Fig. 1</b>. | ||
</p> | </p> | ||
| Line 312: | Line 312: | ||
<p> | <p> | ||
To place <b><i>C. elegans</i> </b>on the plate, different cuts were made on a fresh plate of NGM, the resulting small pieces were placed in the exact | To place <b><i>C. elegans</i> </b>on the plate, different cuts were made on a fresh plate of NGM, the resulting small pieces were placed in the exact | ||
| - | center of the 50% -50% plates to determine which side the nematode preferred, one per Petri plate. | + | center of the 50% -50% plates to determine which side of the nematode preferred, one per Petri plate. |
</p> | </p> | ||
<p> | <p> | ||
| Line 321: | Line 321: | ||
</p> | </p> | ||
<p> | <p> | ||
| - | From <b>Table 2 </b> | + | From <b>Table 2 </b>it could rule out many of the attractants that were thought viable. |
</p> | </p> | ||
<p> | <p> | ||
| - | Volatile attractants were not a good choice | + | Volatile attractants were not a good choice to evaporate quickly (which is also limited to the field experiments). |
</p> | </p> | ||
<p> | <p> | ||
| - | Another impediment arose. Once | + | Another impediment arose. Once had already performed the experiments, it was decided that the promoter that will control the production of RNA interference |
in <i>Escherichia coli</i> would be controlled by nitrogen, amino acids had to be discard as attractants; it would modify controlled expression of E.coli. | in <i>Escherichia coli</i> would be controlled by nitrogen, amino acids had to be discard as attractants; it would modify controlled expression of E.coli. | ||
</p> | </p> | ||
| Line 415: | Line 415: | ||
<p> | <p> | ||
Our<i> <b>C. elegans</b></i> strain <b>(N2 strain</b>) has two feeding behaviors: <b>Social and solitary feeding</b>. Social feeding is known as clumping | Our<i> <b>C. elegans</b></i> strain <b>(N2 strain</b>) has two feeding behaviors: <b>Social and solitary feeding</b>. Social feeding is known as clumping | ||
| - | as you can see in this video: <a href="http://www.youtube.com/watch?v=jCNsmcVVUVY | + | as you can see in this video: <a href="http://www.youtube.com/watch?v=jCNsmcVVUVY">http://www.youtube.com/watch?v=jCNsmcVVUVY</a> |
| + | |||
| + | </p> | ||
| + | <p> | ||
| + | Clumping is known to be induced under some conditions <b>like temperature or starving</b>, something that we had had considered during the entire project. | ||
| + | However, it is also known how clumping is controlled from a genetic perspective and the main genes have already been described. We thought we could use | ||
| + | this genetic approximation to control clumping under specific and controlled conditions. <i>(<a href="http://129.85.244.162/bard/pdf week_07_de_bono_bargmann_natural_variation_in_receptor_modifies_social_behavior_and_food_response_cell_1998.pdf | ||
| + | ">view “</i>Natural variation in a neuropeptide Y receptor | ||
| + | homolog modifies social behavior and food response in C. elegans.<b>”</b> Bono M, Bargmann CI</a>). | ||
| + | </p> | ||
| + | <p> | ||
| + | <h3>How do we induce clumping?</h3> | ||
| + | Our initial thought was to interfere the principal gene involved in this route: <b>NPR-1</b>. It is known that mutations in <b>NPR1</b> convert a solitary | ||
| + | strain into a social strain. Another option we finally chose was <b>FLP-21</b>, a gene that positively regulates <b>NPR-1</b>. We selected this one instead | ||
| + | because it is not involved in so many vital processes. <b>FLP-21</b> encodes a single FMRF amide-related neuropeptide that serves as a ligand for<b>Npr-1</b>, a G protein-coupled receptor that regulates social versus solitary feeding behavior in several <i>Caenorhabditis </i>species (see <b>fig. 1</b>). | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/0/04/Vb_clumping_1.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | So, this would be the final process: engineered<b> <i>E.coli</i> </b>that expresses <b>FLP-21</b>, whom <b>RNA will interfere </b>with the one codified by | ||
| + | the worm because of the ingestion of bacteria. The formation of a complex of dsRNA will induce the elimination of <b>Flp-21 </b>transcripts by the RNA | ||
| + | silencing pathway, which consequence is the induction of clumping (<b>see figure 2)</b>. We also had to take into account that the induction of clumping by | ||
| + | this mechanism is almost immediate, in comparison with natural clumping which takes longer depending on the external factors. | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f8/Vb_clumping_2.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | <h3>Why do we induce clumping for?</h3> | ||
| + | |||
| + | The main objective of the synthetic symbiosis that we designed was to detect hotspots of interest in irregular substrates and the induction of clumping was a good tool for it. With this natural mechanism, which we were going to control, <b>worms kept in the desired places</b>, then <b>bacteria transported by <i>C. elegans </i></b>(<i>Pseudomonas putida)</i> <i> </i><b>were concentrated </b>where their action was needed, in order to generate value-added products such as bioplastic PHA and the increase of worms in a specific location <b>improved the image-based detection </b>mechanism (<a href="https://2013.igem.org/Team:Valencia_Biocampus/Devices">go to Devices section</a>) | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <h3>Controlling the mechanism</h3> | ||
| + | |||
| + | The social feeding behavior needed to be controlled; it might be induced by conditions of interest as a tool for detecting hotspots in irregular substrates. We decided that <b>fatty acids </b>were going to be the inductors of the promoter (<b>fadBp</b>) that regulated the expression in the FLP-21 | ||
| + | antisense sequence, in order to induce RNA interference (<b>Fig.3)</b>. In the fatty acid rich media where this mechanism was activated, bacteria got off | ||
| + | the worm, so this construction allows the induction of clumping in the spots where a biotechnological process will be carried out. | ||
| + | </p> | ||
| + | |||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/9/93/Vb_clumping_3.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <h3>Choosing the right fatty acid</h3> | ||
| + | The <b><i>E. coli’s </i>biobrick<i> </i>promoter is induced by fatty acids</b>, but those also induce the promoter of <i>Pseudomonas putida </i>in order to | ||
| + | produce PHA. Some investigations showed that the induction <b>of fadBp promoter </b>by <b>oleic acid </b>is the most efficient (<a href="http://www.ncbi.nlm.nih.gov/pmc/articles/PMC216235/?page=1"><i>view “</i>Regulation of | ||
| + | fatty acid degradation in Escherichia coli: analysis by operon fusion” Clark D. et al</a>) is | ||
| + | higher when the fatty acid used is <b>oleic acid </b>but this is a long chained compound and that could affect the production of bioplastic (PHA). We | ||
| + | decided to test the growth of <i>E. coli </i>and the production of bioplastic by <b><i>P. putida</i></b><i> </i>using PHA media with oleic acid and | ||
| + | octanoic acid in order to get the best results in both activities. | ||
| + | </p> | ||
| + | <p> | ||
| + | Both species of microorganisms where grown in PHA production media with different concentrations of oleic and octanoic acids, maintaining a global | ||
| + | concentration of fatty acids of 8mM, because it was the one where we found the highest growth of both <i>E. coli </i>and <i>P. putida. </i> <b>Table 2 </b> | ||
| + | (below) summarizes the assays done. | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/d/d1/Vb_clumping_4.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | The results of this assay showed that <i>E. coli </i>growth was better on PHA production media + 8mM oleic acid and the <i>P. putida </i>production of | ||
| + | bioplastic was acceptable in this conditions. However, the highest production of bioplastic was on PHA production media+ 8mM octanoic acid one. So then, we | ||
| + | made a <b>Colonization assay </b>and finally we determined that we were going to continue working using the PHA production media + 8mM oleic acid. Also, | ||
| + | the ODs for plating both organisms were stablished as a consequence of this experiment. The “<b>Choosing the right fatty acid” </b>and the “<b>Colonization” </b>assays are explained at <a href="#4" data-liquidslider-ref="slider-id"><b>Building the bioplastic </b>section.</a> </b> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <h3> Observing clumping induced by fatty acid rich media </h3> | ||
| + | |||
| + | We could see how clumping was done induced by the presence of <b>8mM of oleic acid </b>(2.58 µl/ml ) which regulated the <b>FadBp promoter </b>of <b><i>E. coli</i></b><i> </i>and as a consequence the expression the complementary sequence to the worms’ <b>FLP-21 </b>transcript, producing interference | ||
| + | by RNA. In the plates there was a high concentration of <i>E. coli </i>from the serial centrifugations preculture 4ml <b>XL1-Blue</b>, 2 min at maximum rpm | ||
| + | (2 times). The following images show the change of behavior of <i>C. elegans, </i>which at first were eating alone and then they were doing it in groups as | ||
| + | it was planned <b>(Fig. 4-6).</b> | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/4/4d/Vb_clumping_5.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | |||
| + | </div> | ||
| + | <div> | ||
| + | <h2 class="title"><img src="https://static.igem.org/mediawiki/2013/9/97/White_building_icon.png" alt="" class="title-icon" />The Building</h2> | ||
| + | <h1 class="title2 building-color"><img src="https://static.igem.org/mediawiki/2013/9/97/White_building_icon.png" alt="" class="title2-icon building-bg" />The Building</h1> | ||
| + | |||
| + | <p> | ||
| + | We decided to include in the mechanism design a regulated production of a value-added product in the hotspots of interest were C. elegans would be | ||
| + | attracted to. We got proffit of the natural capacity of Pseudomonas putida of producing bioplastic PHA (polyhydroxyalkanoates) by bacterial fermentation of | ||
| + | sugar or lipids (fatty acids in this case), because we found that the directed production of this versatile material was interesting due to its easy | ||
| + | detection (complexes of PHA with red Nile are fluorescent), its bright future in the field of biomaterials and because we were using this natural ability | ||
| + | of bacteria as a tool. | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <h3>The mechanism</h3> | ||
| + | |||
| + | A cluster of genes is responsible of the production of PHA, the <b>phaC operon </b>of <b><i>P. putida </i></b>which consists of 4 ORFs that are transcribed | ||
| + | in the same direction: phaC1 and phaC2 genes, codify for two PHA sintases; phaZ gene, codifies for a despolimerase; and phaD gene, that codifies for a | ||
| + | protein of the TetR family. In the opposite direction two genes that codify for fasinas (phaF and phaI) and structural proteins are found <b>(Fig.1). </b> | ||
| + | </p> | ||
| + | |||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f8/Vb_building_1.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <h3>The role of the operon in our project</h3> | ||
| + | |||
| + | Our 2013 project is a proof of concept of the benefits that an artificial synthetic symbiosis between bacteria and nematodes can offer. The roles <i>Pseudomonas</i> play are two: firstly, they have been engineered to “ride” on <i>C. elegans</i> by the formation of a biofilm, but not stuffed with that, under the promoter glnA we stimulated the production of PHA, a value-added product that can be widely applied, for example: | ||
| + | <ul> | ||
| + | <li> | ||
| + | For short disposable packaging items (personal hygiene products, surgical clothes). | ||
| + | </li> | ||
| + | <li> | ||
| + | In upholstery. | ||
| + | </li> | ||
| + | <li> | ||
| + | In the photographic and printing industry | ||
| + | </li> | ||
| + | <li> | ||
| + | For textile industry since PHA can be processed into fibers. | ||
| + | </li> | ||
| + | <li> | ||
| + | For biofuel production from PHA obtained from sewage sludge. This would combine two major advantages: the wastewater treatment and the generation of | ||
| + | energy. | ||
| + | </li> | ||
| + | </ul> | ||
| + | </p> | ||
| + | <p> | ||
| + | <h3>Choosing the right media</h3> | ||
| + | |||
| + | On the plates we were going to have both <i>P. putida </i>and <i>E. coli </i>and their promoters’ response towards fatty acids was different, so we had to | ||
| + | make a media where <b><i>E. coli </i></b>could induce<b> clumping </b>and <b><i>P. putida </i></b>the <b>production of bioplastic</b><i>. </i>In <b><i>E. coli, </i></b>the<b><i> </i></b>briobrick promoter for the expression of <b>FLP-21 iRNA </b>engineered in order to induce the social feeding | ||
| + | behavior of<i> C. elegans </i><b>(Clumping) </b><b>is induced by fatty acids</b>, but not all the fatty acids are able to induce the same level expression | ||
| + | (<b>Fig. 2</b>); <b>oleic acid </b>produces the highest activation of the promoter. | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f1/Vb_building_2.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | Whereas the <b>standard medium </b>for the <b>production of PHA </b>by <b><i>Pseudomonas putida</i> </b>(composition reflected in <b>Fig.3</b>) employs <b>octanoic acid </b>(a short-chain fatty acid) as inducer. This fact made necessary to check if the modification of the fatty acid used, octanoic by | ||
| + | oleic, would modify the bioplastic (PHA) production. | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/0/0d/Vb_building_3.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | Otherwise, the medium for PHA production is tuned for the development of <i>Pseudomonas putida</i>, but not for the <i>E.coli</i>, so we had to test if | ||
| + | this bacteria was able to grow in it. When we did this checking, we realized that the development of these bacteria did not occur in the PHA production | ||
| + | media. However, with the addition of acetate (carbon source), the removal of the iron salt (added to the media to avoid the synthesis of a <span class="siderophore-tooltip">siderophore</span> by <i>Pseudomonas sp.</i>) and, due to serendipity, with the double | ||
| + | concentration of trace elementes, we got the media for the enteric bacteria growth. | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <h3>Choosing the right fatty acid</h3> | ||
| + | Firstly, we tried to grow <i>E. coli </i>in the new PHA production media with different concentrations of octanoic acid. The same assays were done in | ||
| + | parallel with <i>P. putida </i>in order to check which were the conditions where both microorganisms could grow and <i>P. putida </i>could produce enough | ||
| + | quantity of bioplastic. | ||
| + | </p> | ||
| + | <p> | ||
| + | After overnight incubation, the cultures were washed using sterile PBS and ODs<sub>600</sub> were measured (<b>Fig.4.</b>). We also <b>checked the PHA production </b>by centrifuging the precultures, resuspending the pellet on PBS and adding Red Nile dissolved in DMSO, we could see the | ||
| + | emission of fluorescence due to the interaction between the Nile red and the PHA through an UV transilluminator <b>(Fig.5)</b>. By the results of the | ||
| + | assays we chose to work with a <b>8mM</b> concentration of fatty acids (<b>Fig.4</b>). | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/d/dc/Vb_building_4.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | We also <b>checked the PHA production </b>by centrifuging the precultures, resuspending the pellet on PBS and adding Red Nile dissolved in DMSO, we could | ||
| + | see the emission of fluorescence due to the interaction between the Nile red and the PHA through an UV transilluminator <b>(Fig.5)</b>. By the results of | ||
| + | the assays we chose to work with a <b>8mM</b> concentration of fatty acids (<b>Fig.4</b>). | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/c/c9/Vb_building_5.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | At this point we found that <i>E. coli </i>and <i>P. putida </i>could grow at 8mM of octanoic acid and that bioplastic (PHA) is also produced. However, | ||
| + | because <i>E. coli </i>grows better in <b>oleic acid </b>and we wanted to check if <b><i>P. putida </i></b>could produce PHA if its phaC operon was induced | ||
| + | by it, we grew both bacteria in <b>different concentrations of octanoic and oleic acid but maintaining a constant concentration of fatty acids of 8mM</b>. | ||
| + | The assays done are explained in the table below (<b>Fig.6</b>). | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/6/6a/Vb_building_6.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | We saw that <i>E. coli </i>had grown better with the increasing of oleic acid, getting at its maximum on experiment 9, even so, the quantity of pellet | ||
| + | corresponding to 1 mL of preculture was scarce (and not visible at experiments 1 to 5). <i>E. coli </i>needed more time to grow in the PHA production | ||
| + | media. | ||
| + | </p> | ||
| + | <p> | ||
| + | <i>P. Putida</i> | ||
| + | had grown too, however we wanted to measure the production of bioplastic, we measured the ODs<sub>600</sub> from the precultures of <i>P. putida </i>by | ||
| + | centrifugating 2 mL of them and then washing them on 1 mL of PBS and resuspending the pellet in 500 μ<b>l </b>of PBS<b>. </b> Then, dilutions on PBS were | ||
| + | adjusted to the lowest OD<sub>600</sub><sub>,</sub>corresponding to the experiment number 9 (8mM oleic acid). The growth of <i>P. putida </i>lower in PHA | ||
| + | production media with a concentration of 8mM of oleic acid (<b>Fig. 7-8)</b>. | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/9/9c/Vb_building_7.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | The results showed that the growth of <b><i>E. coli </i></b>on 8mM of oleic acid was better than in any other condition, then <b>experiment 9 </b> | ||
| + | conditions would be the best ones for the induction of <b>clumping. </b>However, even <b><i>P. putida </i></b>had produced bioplastic at all oleic acid | ||
| + | concentrations, the highest production levels corresponded to <b>experiment 1 </b>conditions, which means in absence of oleic acid, just on 8mM of octanoic | ||
| + | acid. Because of that, next assays were done following the conditions of <b>experiment 1 and 9</b> (<b>Fig.9</b>). | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/2/2f/Vb_building_8.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | After the optimization of the media, kinetics of the production of PHA and biomass generation were realized in relation to the time (<b>Fig.10</b>). | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/8/8c/Vb_building_9.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | <div> | ||
| + | <h2 class="title"><img src="https://static.igem.org/mediawiki/2013/2/20/White_finalresult_icon.png" alt="" class="title-icon" />Pre-field test</h2> | ||
| + | <h1 class="title2 finalresult-color"><img src="https://static.igem.org/mediawiki/2013/2/20/White_finalresult_icon.png" alt="" class="title2-icon finalresult-bg" />Pre-field test</h1> | ||
| + | |||
| + | <p> | ||
| + | <h3>Final Test: PHA Production in an Heterogeneous Substrate (soil)</h3> | ||
| + | Finally, we designed an assay in which all the activities presented till this moment were carried out by the synthetic symbiosis that we have been working | ||
| + | with. In this experiment we achieve the goal of our project<b>: the detection of hotspots of interest in irregular substrates and the production of bioplastic (PHA) in those places</b>. <u>Our project works in a pre-field test!</u> | ||
| + | </p> | ||
| + | <p> | ||
| + | A soil ground was prepared with an artificially distributed substrate-rich medium in discrete points (<b>Fig.1</b>). Once the medium was ready, we | ||
| + | inoculated the soil with a <b><i>C. elegans-Pseudomonas </i></b>suspension and with transformed <b><i>E. coli </i></b>expressing the iRNA of interest. As a | ||
| + | control, we took a picture of the soil ground the first day the microorganisms were added by exposing it to UV light (<b>Fig.2</b>). After a day we added | ||
| + | Nile red to reveal the PHA production and to see if the experiment had finally worked (<b>Fig. 3</b>). It is possible to see bioplastic nodules which are | ||
| + | red when combined with Nile red. | ||
| + | </p> | ||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/d/d0/Vb_finalresult_1.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | <p> | ||
| + | In addition, we took an electron microscopy image of one of the discrete points of medium (<b>Fig. 4</b>) to study what organisms were actually near the | ||
| + | hotspots. More electron microscopy images were taken to demonstrate if <i>C. elegans</i> dragged <i>Pseudomonas</i> to the points of interest ( <b>Fig. 4)</b>. | ||
| + | </p> | ||
| + | |||
| + | <p style="text-align:center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/d/d6/Vb_finalresult_2.png" alt="" style="width:700px" /> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | We finally were able to conclude that our synthetic consortium worked: Worms managed to reach the hotspots of interest, the worms dragged <i>Pseudomonas </i>with them and <i>Pseudomonas</i> finally made bioplastic detected be adding Nile red. | ||
| + | </p> | ||
| + | <p> | ||
| + | In addition, we took an electron microscopy image of one of the discrete points of medium (<b>Fig. 4</b>) to study what organisms were actually near the | ||
| + | hotspots. More electron microscopy images were taken to demonstrate if <i>C. elegans</i> dragged <i>Pseudomonas</i> to the points of interest ( <b>Fig. 4 [right] </b>). | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </div> | ||
| + | <div class="tab-pane fade" id="Parts"> | ||
| + | </html> | ||
| + | |||
| + | == Parts == | ||
| + | <html> | ||
| + | <p> | ||
| + | These are the BioBricks we have designed, constructed, and characterized. We have submitted them to the <a href="http://parts.igem.org/Main_Page">Registry of Standard Biological Parts</a> | ||
| + | </p> | ||
| + | <br/> | ||
| + | <!--An important aspect of the iGEM competition is the use and creation of standard biological parts. Each team will make new parts during iGEM and will place them in the [http://partsregistry.org Registry of Standard Biological Parts]. The iGEM software provides an easy way to present the parts your team has created . The "groupparts" tag will generate a table with all of the parts that your team adds to your team sandbox. Note that if you want to document a part you need to document it on the [http://partsregistry.org Registry], not on your team wiki. | ||
| + | |||
| + | Remember that the goal of proper part documentation is to describe and define a part such that it can be used without a need to refer to the primary literature. The next iGEM team should be able to read your documentation and be able to use the part successfully. Also, you should provide proper references to acknowledge previous authors and to provide for users who wish to know more. | ||
| + | <groupparts>iGEM013 Valencia_Biocampus</groupparts> | ||
| + | |||
| + | --> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <br/> | ||
| + | <table class="table-modeling" border="0" style="background-color: #FFFFFF;" width="950"> | ||
| + | <thead> | ||
| + | <tr> | ||
| + | <th> Works? </th> | ||
| + | <th> Name </th> | ||
| + | <th> Type </th> | ||
| + | <th> Description </th> | ||
| + | <th> Designer </th> | ||
| + | <th> Length </th> | ||
| + | <th> Fav </th> | ||
| + | </tr> | ||
| + | </thead> | ||
| + | <tr> | ||
| + | <td><img class="table-check" src="https://static.igem.org/mediawiki/2013/0/01/CheckIcon.gif" alt="Yes" height="24" width="24"/></td> | ||
| + | <td> <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1112000">BBa_K1112000</a> </td> | ||
| + | <td> Regulatory </td> | ||
| + | <td> fadB promoter + FLP-21 iRNA </td> | ||
| + | <td> Pedro Luis Dorado Morales </td> | ||
| + | <td> 344 </td> | ||
| + | <td> <img src="https://2013.igem.org/common/tablesorter/themes/groupparts/heart13.gif" alt="" /></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><img class="table-cross" src="https://static.igem.org/mediawiki/2013/0/01/CheckIcon.gif" alt="Yes" height="24" width="24"/></td> | ||
| + | <td> <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1112001">BBa_K1112001</a> </td> | ||
| + | <td> Coding </td> | ||
| + | <td> pGlnA + hmsHFRS operon </td> | ||
| + | <td> Alba Iglesias Vilches </td> | ||
| + | <td> 6342</td> | ||
| + | <td> </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td><img class="table-check" src="https://static.igem.org/mediawiki/2013/0/01/CheckIcon.gif" alt="Yes" height="24" width="24"/></td> | ||
| + | <td> <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1112002">BBa_K1112002</a> </td> | ||
| + | <td> Coding </td> | ||
| + | <td> Cluster PHA </td> | ||
| + | <td> Alba Iglesias Vilches </td> | ||
| + | <td> 4978</td> | ||
| + | <td> </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br/> | ||
| + | |||
| + | <p> | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1112000"><h3>BBa_K1112000: fadB promoter + FLP-21 iRNA</h3></a> | ||
| + | |||
| + | This construction is made up by a fatty acid-sensitive promoter that directs the transcription of an iRNA responsible for the social or solitary behavior of <i>C.elegans</i>. | ||
| + | |||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1112001"><h3>BBa_K1112001: pGlnA + hmsHFRS operon</h3></a> | ||
| + | We reused our nitrogen sensitive promoter from one of our last year constructs and we employ it to control the expression on the operon that triggers the formation of a biofilm over <i>C.elegans</i>. | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1112002"><h3>BBa_K1112002: cluster PHA</h3></a> | ||
| + | This BioBrick contains the complete natural sequence that induces the production of PHA in <i>Pseudomonas putida</i> (KT2440). | ||
| + | </p> | ||
| + | |||
| + | </div> | ||
| + | <br/> | ||
| + | </html> | ||
| + | |||
| + | |||
| + | <html> | ||
| + | <script type="text/javascript"> | ||
| + | $(document).ready(function() { | ||
| + | // cache the id | ||
| + | var navbox = $('#myTab'); | ||
| + | // activate tab on click | ||
| + | navbox.on('click', 'a', function (e) { | ||
| + | // add a hash to the URL when the user clicks on a tab | ||
| + | history.pushState(null, null, $(this).attr('href')); | ||
| + | var $this = $(this); | ||
| + | // prevent the Default behavior | ||
| + | e.preventDefault(); | ||
| + | // set the hash to the address bar | ||
| + | window.location.hash = $this.attr('href'); | ||
| + | // activate the clicked tab | ||
| + | $this.tab('show'); | ||
| + | //return false; | ||
| + | }) | ||
| + | |||
| + | // if we have a hash in the address bar | ||
| + | if(window.location.hash){ | ||
| + | // show right tab on load (read hash from address bar) | ||
| + | $('a[href="'+window.location.hash+'"]').tab('show'); | ||
| + | } | ||
| + | |||
| + | // navigate to a tab when the history changes | ||
| + | window.addEventListener("popstate", function(e) { | ||
| + | var activeTab = $('[href=' + location.hash + ']'); | ||
| + | if (activeTab.length) { | ||
| + | activeTab.tab('show'); | ||
| + | } else { | ||
| + | $('.nav-tabs a:first').tab('show'); | ||
| + | } | ||
| + | }); | ||
| + | }); | ||
| + | </script> | ||
| + | |||
| + | </html> | ||
Revision as of 16:00, 4 October 2013
Project Overview
Why E.coli
Escherichia coli is a model organism widely use in fields as microbiology, molecular biology and genetics. Result of that is the great range of genetic manipulation techniques that can be found related to this Gram negative bacterium. There is also a lot of information about the biochemistry and genetics of this microorganism. All this allows to work easily with it in the lab and often perform assays testing different conditions that will be tried out in other organisms unstudied in such depth. Moreover, E.coli is the main food of C.elegans, organism that is able to incorporate nucleic acids from the bacteria, RNA, to its cells (Mello et al., 2004). This is the usual mechanism of transformation of the worm, introducing exogenous genetic material from the bacteria altering the expression of the worm’s genome in order to make modifications of interest.
Controlling the mechanism
The role of E.coli in our project is to carry out the synthesis of an iRNA for inducing the social feeding behavior of Caenorhabditis elegans , the clumping. To achieve this, we cloned the biobrick Bba_K1112000 in E. coli, XL1-Blue strain (fig. 1).

Fig. 1.
- pSB1C3 is a high copy number plasmid (RFC [10]) carrying chloramphenicol resistance.
- The replication origin is a pUC19-derived pMB1 (copy number of 100-300 per cell).
- pSB1C3 has terminators bracketing its MCS which are designed to prevent transcription from *inside* the MCS from reading out into the vector. The efficiency of these terminators is known to be < 100%. Ideally we would construct a future set of terminators for bracketing a MCS that were 100% efficient in terminating both into and out of the MCS region.
This construction consists of the E.coli fadA promoter, which is activated in the presence of fatty acids (Clark, 1981), and the antisense sequence of the mRNA FLP-21 from C.elegans which encodes a protein involved in the solitary feeding behaviour of the worm, the formation of the dsRNA complex inhibits the expression of this protein, inducing the social feeding behavior (fig. 2).

Pseudomonas putida and PHA production
Pseudomonas putida is a gram-negative bacterium that is found in most soil and water habitats where there is oxygen. Its diverse metabolism and its capacity to break down organic harmful solvents (it has most genes involved in degrading aromatic or aliphatic hydrocarbons) in contaminated soils make this microorganism irreplaceable for research studies in the field of bioremediation but also for biosynthesis of value-added products. In addition, Pseudomonas putida has several strains including KT2440, the one we have worked with. This strain can colonize plant roots, from which they take nutrients, while at the same time it offers protection to the plant from pathogens.
For example, it is capable of converting styrene oil into the biodegradable plastic PHA. This helps to degrade the polystyrene foam which
was thought to be non-biodegradable. Styrene is a major environment toxic pollutant released from industrial sites. The conversion to PHA allows the cure
of styrene pollution but it is also beneficial to society because of its applications in tissue engineering.
PHA is also environmental friendly and has a long self-life therefore it is also used in everyday items. Unlike styrene, PHA can break down in soil or water.
Within Pseudomonas putida, PHA accumulates under unbalanced growth conditions as a means of intracellular storage, storing excess carbon and energy. These PHA polymers are synthesized by enzyme PHA synthase which is bound to the surface of the PHA granules and uses coenzyme A thioesters of hydroxyalkanoic acids as substrates.

The role of P. putida in the Synthetic symbiosis that we have designed is to be carried by C. elegans to hotspots of interest where would produce bioplastic PHA.
An overview about our nematode
One of the main characters of our work is a nematode known as Caenorhabditis elegans, from the Rhabditidae family. It was first used as an experimental model in Developmental Genetics studies and nowadays is also used in other fields such as Clinical Biology, Neurobiology and Cell Biology, being a good model to study Alzheimer disease, obesity, diabetes and aging, among others.
Another interesting thing is that it feeds on Escherichia coli. Its “favorite” strain is OP50, although we checked that it’s also able to feed on XL1-Blue strain, the one that we used in all our molecular biology experiments.
Some advantages of C. elegans when compared with other model organisms are:
- Lifespan ranges between 2 and 3 weeks, so experimentation times are reduced.
- Its maintenance and study is cheap and simple (it is transparent, which facilitates microscopic observation).
- It is very small (1 mm), so it is possible to carry out experiments with a huge number of worms in a small Petri dish having a great statistical support.
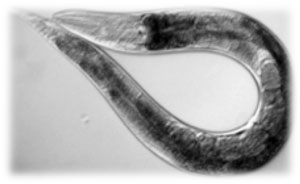
Why use C. elegans as a transport?
When we were considering on creating a new system for the transport of bacteria, we found different key advantages that made the nematode the best option. For example, C. elegans is able to move very fast around solid substrates (soil is, actually, its natural habitat) and also in agar, so it’s very useful for both lab experiments and real-environment tests.
Its movement, in addition to being very fast, has two modes: random and directed.
When there is no attractant in the medium, C. elegans moves doing uncoordinated movements in several directions in what is known as 'random walk'.
The situation changes when there is an attractant in the medium. Here, our 'transport' begins to direct his movements to the focus of the substance (volatile or soluble) which acts as an attractant in a process known as 'chemotaxis' thanks to the amazing smell of our nematode. This allows us to ‘guide’ the nematodes towards defined spots in irregular substrates.
Chemotaxis is the foundation to guide the transport of bacteria and is therefore the focus of experimentation with C. elegans, with the aim of finding the best attractant. This ability makes C. elegans a perfect 'bus' for bacteria. (simuelegans online here)
Moreover, we found two pathogens (Yersinia pestis and Xhenorhabus nematophila) with the ability to form biofilms on C. elegans thanks to the proteins of the operon hmsHFRS. When genetically-engineered strains of commonly used bacteria such as Escherichia coli or Pseudomonas putida express this hmsHFRS operon, they have the 'ticket' to travel: they are able to adhere to the worm’s surface by means of forming a synthetic biofilm.
Expanding our knowledge about C. elegans...
Once we knew that C. elegans was the best option, we began to discover interesting things for further study.
There are several strains of our nematode. The one that drew our attention was called 'N2', which had a fairly interesting behavior: under normal conditions, it eats individually, whereas under certain conditions (such as starvation), a social feeding behavior known as “clumping” is induced. But this phenomenon can also be induced if the expression of some particular genes is interfered. This fact gave us the opportunity to develop the first artificial symbiosis between worms and bacteria, based on the manipulation of the behaviour of C. elegans by simply nourishing it with transformed E.coli able to synthesize the iRNA.
With it, while C. elegans acts as transport, bacteria return the favour giving it the ability to eat in company.

Results
Parts
These are the BioBricks we have designed, constructed, and characterized. We have submitted them to the Registry of Standard Biological Parts
| Works? | Name | Type | Description | Designer | Length | Fav |
|---|---|---|---|---|---|---|
| BBa_K1112000 | Regulatory | fadB promoter + FLP-21 iRNA | Pedro Luis Dorado Morales | 344 |  |
|
| BBa_K1112001 | Coding | pGlnA + hmsHFRS operon | Alba Iglesias Vilches | 6342 | ||
| BBa_K1112002 | Coding | Cluster PHA | Alba Iglesias Vilches | 4978 |
BBa_K1112000: fadB promoter + FLP-21 iRNA
This construction is made up by a fatty acid-sensitive promoter that directs the transcription of an iRNA responsible for the social or solitary behavior of C.elegans.
BBa_K1112001: pGlnA + hmsHFRS operon
We reused our nitrogen sensitive promoter from one of our last year constructs and we employ it to control the expression on the operon that triggers the formation of a biofilm over C.elegans.
BBa_K1112002: cluster PHA
This BioBrick contains the complete natural sequence that induces the production of PHA in Pseudomonas putida (KT2440).
 "
"


























