Team:Heidelberg/Templates/DelH week15
From 2013.igem.org
m |
m |
||
| Line 1: | Line 1: | ||
| + | |||
==05-08 - 13-08-13 == | ==05-08 - 13-08-13 == | ||
===Generation of DelH Plasmid 01-08=== | ===Generation of DelH Plasmid 01-08=== | ||
| Line 45: | Line 46: | ||
Expected band: 663 bp | Expected band: 663 bp | ||
<br/> | <br/> | ||
| - | |||
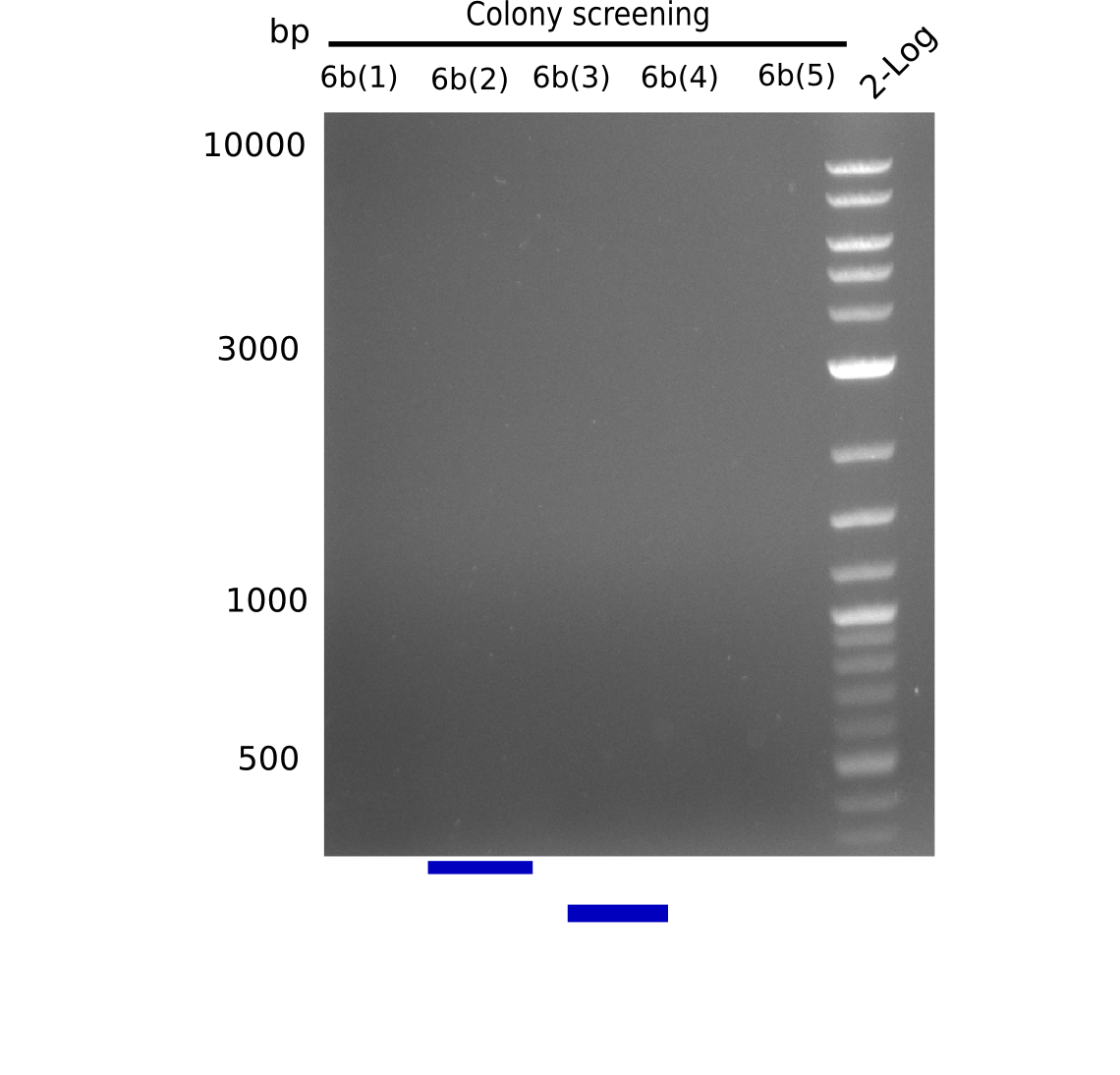
[[File:Heidelberg_20130805 colonyPCR6b.png|200px|thumb|right|'''Fig.15.2''' Gel of picked colonies with insert DelH-BB (loaded 20 µL of PCR) <br> ''l1-5:'' 5 seperate colonies which were together in 3a PCR earlier, ''l6:'' 2log <br> no bands = no colony positive]] | [[File:Heidelberg_20130805 colonyPCR6b.png|200px|thumb|right|'''Fig.15.2''' Gel of picked colonies with insert DelH-BB (loaded 20 µL of PCR) <br> ''l1-5:'' 5 seperate colonies which were together in 3a PCR earlier, ''l6:'' 2log <br> no bands = no colony positive]] | ||
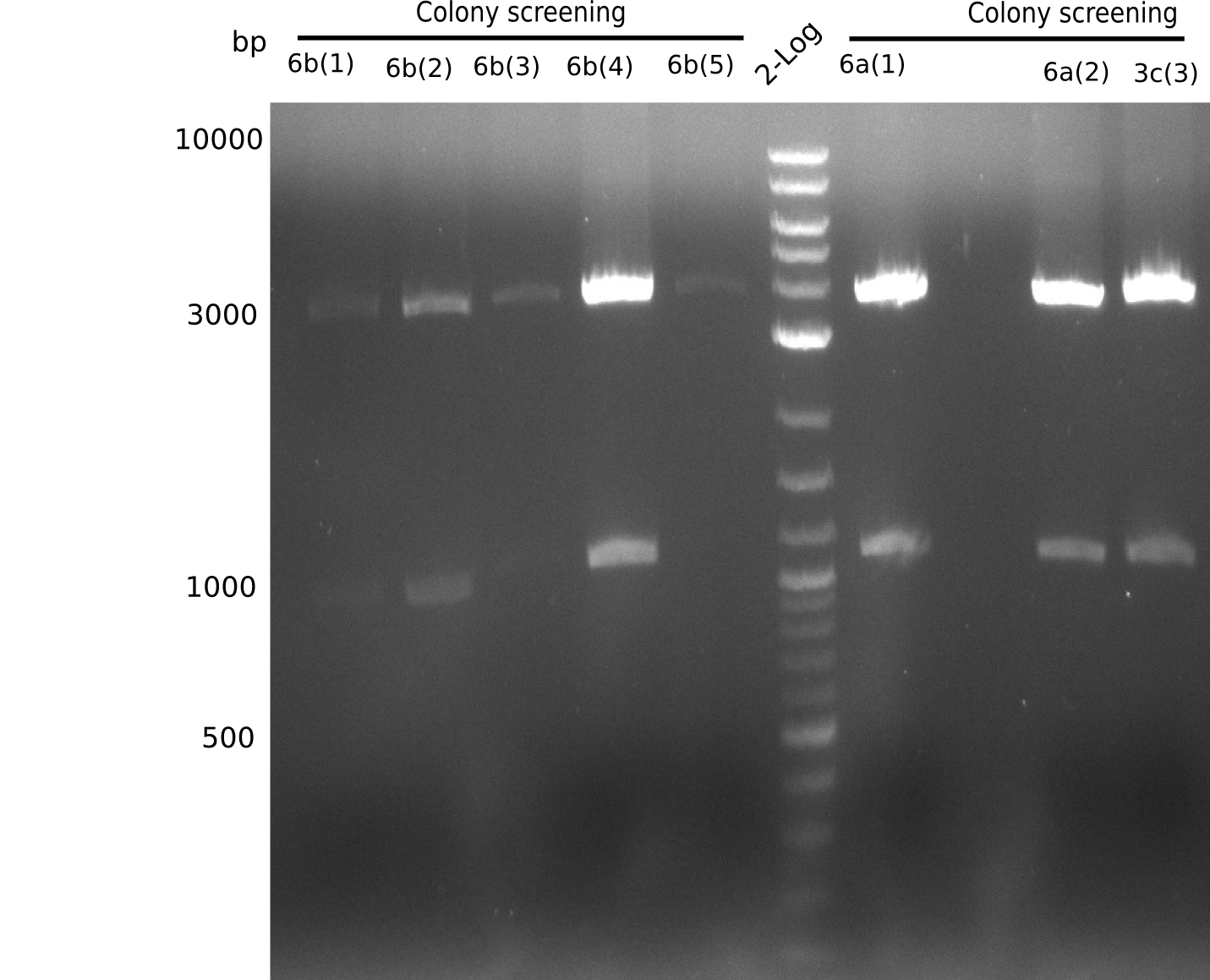
| - | <div style="clear: | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130805 colonyPCR3c-4a.png|200px|thumb|right|'''Fig.15.1''' Gel of picked colonies with insert DelH-BB (loaded 20 µL of PCR) <br> ''l1-5:'' 5 seperate colonies which were together in 3a PCR earlier, ''l6:'' 2log, ''l7-11:'' 5 seperate colonies which were together in 3a PCR earlier, <br> no bands = no colony positive]]</div> |
| + | |||
| + | |||
None of the gels shows expected band. | None of the gels shows expected band. | ||
:=> None of the colonies is positive. | :=> None of the colonies is positive. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====Miniprep==== | ====Miniprep==== | ||
Of colonies 6b(1)-6b(5), 6a(1), 6a(2), 3c(3), a miniprep was performed. | Of colonies 6b(1)-6b(5), 6a(1), 6a(2), 3c(3), a miniprep was performed. | ||
| Line 98: | Line 101: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130805 testdigest.png|200px|thumb|right|'''Fig.15.3''' test digest of listed colonies (loaded 20 µL of PCR) <br> ''l1-5:'' 5 seperate colonies of 6b, ''l6:'' 2log, ''l7'' 6a(1), ''l8'' 6a(2), ''l9'' 3c(3) <br> results are shown in table below]] | [[File:Heidelberg_20130805 testdigest.png|200px|thumb|right|'''Fig.15.3''' test digest of listed colonies (loaded 20 µL of PCR) <br> ''l1-5:'' 5 seperate colonies of 6b, ''l6:'' 2log, ''l7'' 6a(1), ''l8'' 6a(2), ''l9'' 3c(3) <br> results are shown in table below]] | ||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
| Line 120: | Line 122: | ||
Possible next steps: | Possible next steps: | ||
:a) Wait for the new strain of ''D. acidovorans'' SHP1 | :a) Wait for the new strain of ''D. acidovorans'' SHP1 | ||
| + | |||
:b) Perform a re-PCR of minipreps (one with BB and one with DelH G0 fragment) | :b) Perform a re-PCR of minipreps (one with BB and one with DelH G0 fragment) | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Generation of Backbone pSB6A1-lacI-mRFP=== | ===Generation of Backbone pSB6A1-lacI-mRFP=== | ||
====Restriction Digest with DpnI==== | ====Restriction Digest with DpnI==== | ||
| Line 147: | Line 150: | ||
=> Again, checking on gel (2 µl + 8 µl 1xloading dye) <br/> | => Again, checking on gel (2 µl + 8 µl 1xloading dye) <br/> | ||
[[File:Heidelberg_20130808 pSB6A1 2log.png|200px|thumb|right|'''Fig.15.4''' checking concentration of purified BB (PSB6A1-lacI-mRFP) (loaded 2 µL of PCR) <br>''l1'' 2log ladder ''l2:'' pSB6A1-lacI-mRFP <br> ''l2'' show expected band at 5 Kb ]] | [[File:Heidelberg_20130808 pSB6A1 2log.png|200px|thumb|right|'''Fig.15.4''' checking concentration of purified BB (PSB6A1-lacI-mRFP) (loaded 2 µL of PCR) <br>''l1'' 2log ladder ''l2:'' pSB6A1-lacI-mRFP <br> ''l2'' show expected band at 5 Kb ]] | ||
| - | + | ||
:=> Concentration at nanodrop was 27.2 ng/µl | :=> Concentration at nanodrop was 27.2 ng/µl | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G0=== | ===Amplification of DelH G0=== | ||
====PCR Conditions G0.W15.A==== | ====PCR Conditions G0.W15.A==== | ||
| Line 195: | Line 199: | ||
<br/> | <br/> | ||
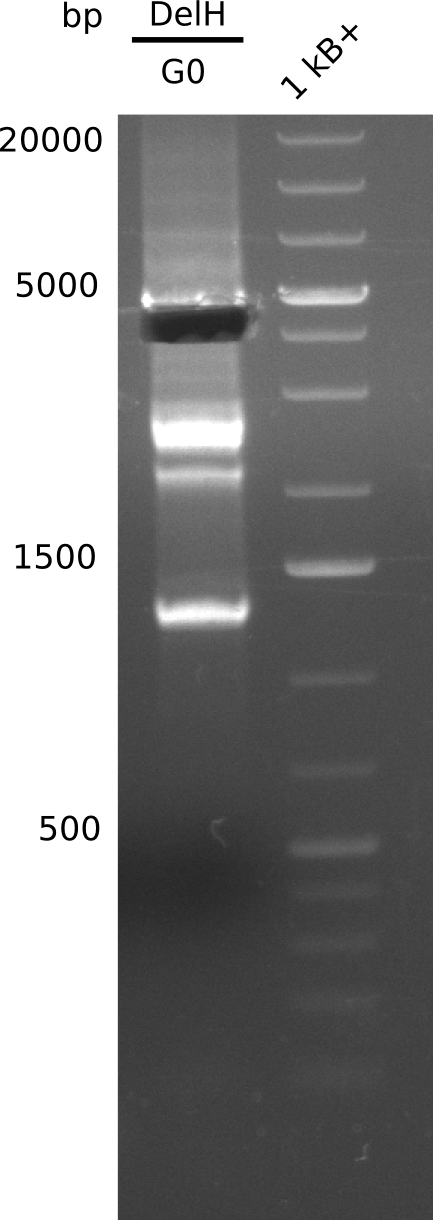
[[File:Heidelberg_20130807 G0.png|200px|thumb|right|'''Fig.15.5''' amplified fragment DelH-complete (loaded 20 µL of PCR) <br>''l1:'' G0, ''l2''1Kb+ ladder <br> no band at 18 Kb only a specific band at 5 Kb, but that is not useful]] | [[File:Heidelberg_20130807 G0.png|200px|thumb|right|'''Fig.15.5''' amplified fragment DelH-complete (loaded 20 µL of PCR) <br>''l1:'' G0, ''l2''1Kb+ ladder <br> no band at 18 Kb only a specific band at 5 Kb, but that is not useful]] | ||
| - | + | ||
Gel does not show the expected band. | Gel does not show the expected band. | ||
:=> Further improve PCR conditions. | :=> Further improve PCR conditions. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====PCR Conditions G0.W15.B==== | ====PCR Conditions G0.W15.B==== | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
| Line 236: | Line 241: | ||
| 1 || 12 || inf | | 1 || 12 || inf | ||
|} | |} | ||
| - | + | ||
* Lid preheated at 98°C | * Lid preheated at 98°C | ||
* No hot start | * No hot start | ||
:=> Becaus it worked well we amplified 3x G0 with the same conditions. | :=> Becaus it worked well we amplified 3x G0 with the same conditions. | ||
| + | <div style="clear:both"></div> | ||
====PCR Conditions G0.W15.C==== | ====PCR Conditions G0.W15.C==== | ||
3x 20 µl PCR reactions were prepared | 3x 20 µl PCR reactions were prepared | ||
| Line 278: | Line 284: | ||
| 1 || 12 || inf | | 1 || 12 || inf | ||
|} | |} | ||
| - | |||
* Lid preheated at 98°C | * Lid preheated at 98°C | ||
* No hot start | * No hot start | ||
| + | <div style="clear:both"></div> | ||
====Result==== | ====Result==== | ||
Expected band: 18 Kb | Expected band: 18 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130808 3xG0 2xG1-2a.png|200px|thumb|right|'''Fig.15. | + | [[File:Heidelberg_20130808 3xG0 2xG1-2a cut.png|200px|thumb|right|'''Fig.15.7''' gel of amplified DelH-fragment (loaded 20 µL of PCR) <br> ''l1-3:''3x G0 (complete fragment amplified with DN11 & HM08), ''l4:'' 1kb+ ladder, ''l5-6:''2x G1/2a (complete fragment amplified with DN11 & HM06) <br> ''l1-3:'' band at 18 Kb was cut out ''l5-6:'' band at 13 Kb was cut out]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130808 3xG0 2xG1-2a.png|200px|thumb|right|'''Fig.15.6''' gel of amplified DelH-fragment (loaded 20 µL of PCR) <br> ''l1-3:''3x G0 (complete fragment amplified with DN11 & HM08), ''l4:'' 1kb+ ladder, ''l5-6:''2x G1/2a (complete fragment amplified with DN11 & HM06) <br> ''l1-3:'' show the expected band at 18 Kb and ''l5-6:'' show the specific band at 13 Kb]]</div> | |
| - | + | ||
| + | |||
Gel shows the expected bands. | Gel shows the expected bands. | ||
:=> Fragments were cut and gel extracted. | :=> Fragments were cut and gel extracted. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G1/2a=== | ===Amplification of DelH G1/2a=== | ||
====PCR Conditions G1/2a.W15.A==== | ====PCR Conditions G1/2a.W15.A==== | ||
| Line 328: | Line 336: | ||
| 1 || 12 || inf | | 1 || 12 || inf | ||
|} | |} | ||
| - | + | ||
* Lid preheated at 98°C | * Lid preheated at 98°C | ||
* No hot start | * No hot start | ||
| - | |||
| - | |||
| - | |||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| + | ====Result==== | ||
| + | [[File:Heidelberg_20130808 3xG0 2xG1-2a cut.png|200px|thumb|right|'''Fig.15.7''' gel of amplified DelH-fragment (loaded 20 µL of PCR) <br> ''l1-3:''3x G0 (complete fragment amplified with DN11 & HM08), ''l4:'' 1kb+ ladder, ''l5-6:''2x G1/2a (complete fragment amplified with DN11 & HM06) <br> ''l1-3:'' band at 18 Kb was cut out ''l5-6:'' band at 13 Kb was cut out]] | ||
| + | <div class="tright" style="clear:none">[[File:Heidelberg_20130808 3xG0 2xG1-2a.png|200px|thumb|right|'''Fig.15.6''' gel of amplified DelH-fragment (loaded 20 µL of PCR) <br> ''l1-3:''3x G0 (complete fragment amplified with DN11 & HM08), ''l4:'' 1kb+ ladder, ''l5-6:''2x G1/2a (complete fragment amplified with DN11 & HM06) <br> ''l1-3:'' show the expected band at 18 Kb and ''l5-6:'' show the specific band at 13 Kb]]</div> | ||
| + | |||
| + | |||
Gel shows the expected bands. | Gel shows the expected bands. | ||
:=> Fragments were cut and gel extracted. | :=> Fragments were cut and gel extracted. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====PCR Conditions G1/2a.W15.B==== | ====PCR Conditions G1/2a.W15.B==== | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
| Line 375: | Line 386: | ||
| 1 || 12 || inf | | 1 || 12 || inf | ||
|} | |} | ||
| - | + | ||
* Lid preheated at 98°C | * Lid preheated at 98°C | ||
* No hot start | * No hot start | ||
| + | <div style="clear:both"></div> | ||
====Result==== | ====Result==== | ||
Expected band: 13 Kb | Expected band: 13 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130808 3xG0 2xG1-2a.png|200px|thumb|right|'''Fig.15. | + | [[File:Heidelberg_20130808 3xG0 2xG1-2a cut.png|200px|thumb|right|'''Fig.15.9''' gel of amplified DelH-fragment (loaded 20 µL of PCR) <br> ''l1-3:''3x G0 (complete fragment amplified with DN11 & HM08), ''l4:'' 1kb+ ladder, ''l5-6:''2x G1/2a (complete fragment amplified with DN11 & HM06) <br> ''l1-3:'' band at 18 Kb was cut out ''l5-6:'' band at 13 Kb was cut out]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130808 3xG0 2xG1-2a.png|200px|thumb|right|'''Fig.15.8''' gel of amplified DelH-fragment (loaded 20 µL of PCR) <br> ''l1-3:''3x G0 (complete fragment amplified with DN11 & HM08), ''l4:'' 1kb+ ladder, ''l5-6:''2x G1/2a (complete fragment amplified with DN11 & HM06) <br> ''l1-3:'' show the expected band at 18 Kb and ''l5-6:'' show the specific band at 13 Kb]]</div> | |
| - | + | ||
| + | |||
Gel shows the expected bands. | Gel shows the expected bands. | ||
:=> Fragments were cut and gel extracted. | :=> Fragments were cut and gel extracted. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G2b=== | ===Amplification of DelH G2b=== | ||
====PCR Conditions G2b.W15.A==== | ====PCR Conditions G2b.W15.A==== | ||
| Line 425: | Line 439: | ||
| 1 || 12 || inf | | 1 || 12 || inf | ||
|} | |} | ||
| - | + | ||
* Lid preheated at 98°C | * Lid preheated at 98°C | ||
* No hot start | * No hot start | ||
| + | <div style="clear:both"></div> | ||
====Result==== | ====Result==== | ||
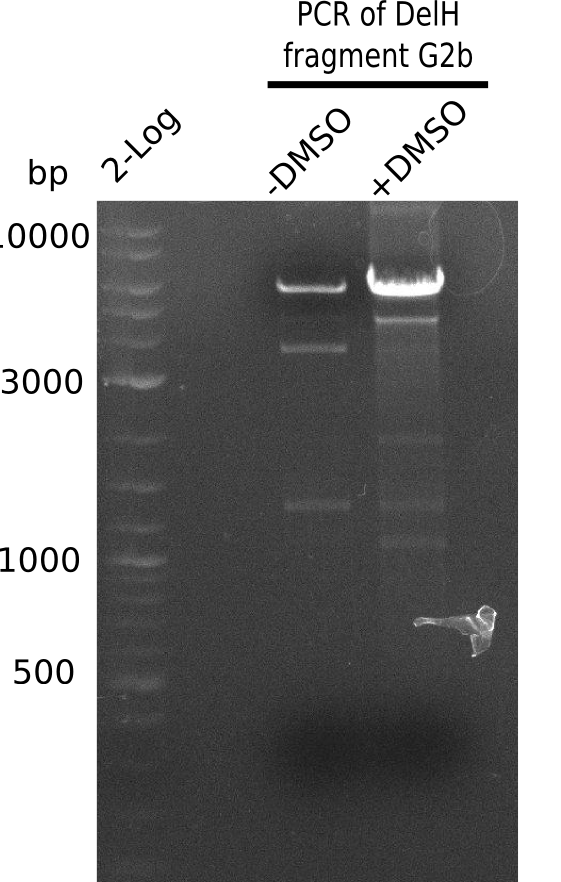
[[File:Heidelberg_20130808 2log 2xDelHG2b.png|200px|thumb|'''Fig. 15.10''': Gel of amplified DelH G2b fragment ''l1:''2log, ''l2-3:''G2b amplified]] | [[File:Heidelberg_20130808 2log 2xDelHG2b.png|200px|thumb|'''Fig. 15.10''': Gel of amplified DelH G2b fragment ''l1:''2log, ''l2-3:''G2b amplified]] | ||
| Line 511: | Line 526: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130810_Colony_PCR_DelH_Gel1_2.png|200|thumb|right|'''Fig.15.12''' screening PCR with DN07 & VF2 (loaded 1 µL of PCR) <br> ''l1:''2log ladder,''l2-17:'' colonies C1, ''l18-24:'' colonies C2, ''l25:''2log ladder <br> None of the colonies showed expected band, instead, all have band at ~6 kb]] | [[File:Heidelberg_20130810_Colony_PCR_DelH_Gel1_2.png|200|thumb|right|'''Fig.15.12''' screening PCR with DN07 & VF2 (loaded 1 µL of PCR) <br> ''l1:''2log ladder,''l2-17:'' colonies C1, ''l18-24:'' colonies C2, ''l25:''2log ladder <br> None of the colonies showed expected band, instead, all have band at ~6 kb]] | ||
| - | + | ||
Gel shows no banda at 663 bp. Instead, they show an unexpected band at ~6 Kb. | Gel shows no banda at 663 bp. Instead, they show an unexpected band at ~6 Kb. | ||
:=> None of the colonies is positive. | :=> None of the colonies is positive. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
====Conoly-PCR Conditions CP.W15.B==== | ====Conoly-PCR Conditions CP.W15.B==== | ||
Picked 20 colonies from plates with 10 µl and 100 µl construct 1 and construct 2 (80 colonies). 4 colonies were picked from the control (10 µl) and control (100 µl). | Picked 20 colonies from plates with 10 µl and 100 µl construct 1 and construct 2 (80 colonies). 4 colonies were picked from the control (10 µl) and control (100 µl). | ||
| Line 593: | Line 608: | ||
[[File:Heidelberg_20130811 SCREENINGpcrA-P.png|thumb|right|'''Fig.15.13''' screening PCR with DN07 & VF2 (loaded 1 µL of PCR) <br>''l1-9:'' colonies A-I, ''l10:''2log ladder, ''l11-17:'' colonies J-P, ''l12:''2log ladder, ''l13-18:'' PCR performed with 6 colonies of the control plate <br> Neither the colonies A-P show the expected band nor the control shows any band]] | [[File:Heidelberg_20130811 SCREENINGpcrA-P.png|thumb|right|'''Fig.15.13''' screening PCR with DN07 & VF2 (loaded 1 µL of PCR) <br>''l1-9:'' colonies A-I, ''l10:''2log ladder, ''l11-17:'' colonies J-P, ''l12:''2log ladder, ''l13-18:'' PCR performed with 6 colonies of the control plate <br> Neither the colonies A-P show the expected band nor the control shows any band]] | ||
:=> New Gibson Assembly will be performed | :=> New Gibson Assembly will be performed | ||
| - | + | ||
Gel shows no banda at 663 bp. | Gel shows no banda at 663 bp. | ||
:=> Neither the colonies A-P show the expected band, nor the control shows any band. | :=> Neither the colonies A-P show the expected band, nor the control shows any band. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Generation of DelH Plasmid 11-08=== | ===Generation of DelH Plasmid 11-08=== | ||
====Gibson Assembly==== | ====Gibson Assembly==== | ||
Revision as of 12:40, 25 October 2013
Contents
|
05-08 - 13-08-13
Generation of DelH Plasmid 01-08
Colony-PCR Conditions CP.W15.A
From colonies inoculated on 04-08
| Reagent | 3c | 3c | 3c | 3c | 3c | 4a | 4a | 4a | 4a | 4a | 6b | 6b | 6b | 6b | 6b | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected length [kb] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | |||
| Named | 3c 1 | 3c 2 | 3c 3 | 3c 4 | 3c 5 | 4a 1 | 4a 2 | 4a 3 | 4a 4 | 4a 5 | 6b 1 | 6b 2 | 6b 3 | 6b 4 | 6b 5 | |||
| Template (1 µl) | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E3c | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E4a | 1 colonies of E6b | 1 colonies of E6b | 1 colonies of E6b | 1 colonies of E6b | 1 colonies of E6b | |||
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | |||
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | |||
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | |||
| ddH2O | 5 µl | 5 µl | 5µl | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl | 5µl | 5 µl | 5 µl | 5 µl | 5 µl | 5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 60 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 60 |
| 65 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
None of the gels shows expected band.
- => None of the colonies is positive.
Miniprep
Of colonies 6b(1)-6b(5), 6a(1), 6a(2), 3c(3), a miniprep was performed.
| Sample | Concentration [ng/µl] | µl avaiblable |
|---|---|---|
| 6b(1) | 33.8 | 18 |
| 6b(2) | 33.4 | 18 |
| 6b(3) | 36.9 | 18 |
| 6b(4) | 59.6 | 18 |
| 6b(5) | 18 | 18 |
| 6a(1) | 60 | 18 |
| 6a(2) | 62.3 | 18 |
| 3c(3) | 40.9 | 18 |
Test Restriction Digest
| Sample | Miniprep [µl] | CutSmart Buffer [µl] | NotI-HF [µl] | H2O [µl] | Total [µl] |
|---|---|---|---|---|---|
| 6b(1) | 15 | 2 | 1 | 3 | 20 |
| 6b(2) | 15 | 2 | 1 | 3 | 20 |
| 6b(3) | 15 | 2 | 1 | 3 | 20 |
| 6b(4) | 10 | 2 | 1 | 8 | 20 |
| 6b(5) | 15 | 2 | 1 | 3 | 20 |
| 6a(1) | 10 | 2 | 1 | 8 | 20 |
| 6a(2) | 10 | 2 | 1 | 8 | 20 |
| 3c(3) | 15 | 2 | 1 | 3 | 20 |
Result
Expected bands: 11,079 bp, 6,201 bp, 3,998 bp, 2,371 bp
| Expected bands | Present bands |
|---|---|
| 11.079 bp | ? |
| 6.201 bp | no |
| 3.998 bp | yes |
| 2.371 bp | no |
in silico PCR of BB shows the following result:
- 2 fragments generated:
- 1: 3.998 bp - From NotI[9] To NotI[4007]
- 2: 1.093 bp - From NotI[4007] To NotI[9]
But an additional band between 1-2 Kb is present!
- => Talk to Advisors, because it isn't what we expected. What could it be?
Possible next steps:
- a) Wait for the new strain of D. acidovorans SHP1
- b) Perform a re-PCR of minipreps (one with BB and one with DelH G0 fragment)
Generation of Backbone pSB6A1-lacI-mRFP
Restriction Digest with DpnI
After consulting the advisors, we decided not to continue the strategy with the old construct and wait with DelH until the new strain of D. acidovorans arrives. Until then, we digest the backbone (which is used also in following experiments) with DpnI to get rid of any remaining template.
| Reagent | Amount [µl] |
|---|---|
| Gel extracted lineralized by PCR (template=10.4 Kb) | 17 |
| DpnI | 1 |
| CutSmart buffer | 2 |
| Total | 20 |
- 1 h incubation at 37°C
- 20 min inactivation of enzyme by heat shock at 80°C for 20 min
Purification
- As template, the PCR of 10,4 PCR, which was digested with DpnI on 06-08, was used.
- Purification was performed with nucleotide removal kit (wrong column was used, but there was some yield at the nanodrop)
=> Again, checking on gel (2 µl + 8 µl 1xloading dye)
- => Concentration at nanodrop was 27.2 ng/µl
Amplification of DelH G0
PCR Conditions G0.W15.A
| Reagent | Amount [µl] |
|---|---|
| Expected length [Kb] | 18.521 |
| Named | G0 |
| Template | 1 µl New strain culture SHP1 (resuspended pellet) |
| Primer fw 10 µM | 1 µl short2 |
| Primer rev 10 µM | 1 µl HM08 |
| Phusion Flash Ready Mix | 10 |
| ddH2O | 5 |
| DMSO | 1 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:45 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Lid preheated at 98°C
- No hot start
Result
Expected band: 18.521 Kb
Gel does not show the expected band.
- => Further improve PCR conditions.
PCR Conditions G0.W15.B
| Reagent | Amount [µl] |
|---|---|
| Expected length [Kb] | 18.521 |
| Named | G0 |
| Template | Picked colony |
| Primer fw 10 µM | 1 µl short2 |
| Primer rev 10 µM | 1 µl HM08 |
| Phusion Flash Ready Mix | 10 |
| ddH2O | 5 |
| DMSO | 1 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:45 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Lid preheated at 98°C
- No hot start
- => Becaus it worked well we amplified 3x G0 with the same conditions.
PCR Conditions G0.W15.C
3x 20 µl PCR reactions were prepared
| Reagent | Amount [µl] |
|---|---|
| Expected length [Kb] | 18.521 |
| Named | G0 |
| Template | Picked colony |
| Primer fw 10 µM | 1 µl short2 |
| Primer rev 10 µM | 1 µl HM08 |
| Phusion Flash Ready Mix | 10 |
| ddH2O | 5 |
| DMSO | 1 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:45 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Lid preheated at 98°C
- No hot start
Result
Expected band: 18 Kb
Gel shows the expected bands.
- => Fragments were cut and gel extracted.
Amplification of DelH G1/2a
PCR Conditions G1/2a.W15.A
| Reagent | Amount [µl] | Amount [µl] |
|---|---|---|
| Expected length [Kb] | 13.083 | 13.083 |
| Named | G1/2a | G1/2a |
| Template | Picked colony | Picked colony |
| Primer fw 10 µM | 1 µl short2 | 1 µl short2 |
| Primer rev 10 µM | 1 µl HM06 | 1 µl HM06 |
| Phusion Flash Ready Mix | 10 | 10 |
| ddH2O | 5 | 4 |
| DMSO | 1 | 0 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:45 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Lid preheated at 98°C
- No hot start
Result
Gel shows the expected bands.
- => Fragments were cut and gel extracted.
PCR Conditions G1/2a.W15.B
| Reagent | Amount [µl] |
|---|---|
| Expected length [Kb] | 13.083 |
| Named | G1/2a |
| Template | Picked colony |
| Primer fw 10 µM | 1 µl short2 |
| Primer rev 10 µM | 1 µl HM06 |
| Phusion Flash Ready Mix | 10 |
| ddH2O | 5 |
| DMSO | 1 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:45 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Lid preheated at 98°C
- No hot start
Result
Expected band: 13 Kb
Gel shows the expected bands.
- => Fragments were cut and gel extracted.
Amplification of DelH G2b
PCR Conditions G2b.W15.A
| Reagent | Amount [µl] | Amount [µl] |
|---|---|---|
| Expected length [Kb] | 5.472 | 5.472 |
| Named | G2b | G2b |
| Template | Picked colony | Picked colony |
| Primer fw 10 µM | 1 µl HM07 | 1 µl HM07 |
| Primer rev 10 µM | 1 µl HM08 | 1 µl HM08 |
| Phusion Flash Ready Mix | 10 | 10 |
| ddH2O | 5 | 4 |
| DMSO | 1 | 0 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 68 | 5 | |
| 72 | 120 | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Lid preheated at 98°C
- No hot start
Result
Summary of Gel Extracted Fragments
Concentrations (by nanodrop) are summarized in the following table and gel picture.
| Fragment | Concentration [ng/µl] |
|---|---|
| G0 | 3.5 |
| G1/2a | 4.9 |
| G2b | 18.6 |
Generation of DelH Plasmid 09-08
Gibson Assembly
We decided two assemble 2 different constructs and one control (only the backbone).
| Construct | G0 [µl] | G1/2a [µl] | G2b [µl] | BB [µl] | 2x Gibson Master Mix [µl] | Final amount [µl] |
|---|---|---|---|---|---|---|
| 1 | 9 | 0 | 0 | 1 | 10 | 20 |
| 2 | 0 | 8 | 1 | 1 | 10 | 20 |
| Control (C) | 0 | 0 | 0 | 1 (+9 ddH2O ) | 10 | 20 |
- Incubated 1 h at 50°C
Electroporation
- Of each Gibson assembly, 5 µl are diluted in 10 µl ddH2O
- The rest of the Gibson assembly is stored at -20°C
Conoly-PCR Conditions CP.W15.A
Picked 8 colonies from plates with 10µl and 100 µl construct 1, and plate with 100 µl of construct 2. 1 additional colony was picked from plate with 10 µl of construct 2 (because there were no more cells) and 1 colony of the control(100µl), and one colony of the control (100µl) with new electrocompetent cells.
- In the following table construct 1 is named C1, and construct 2 = C2 and Control = Co
| Reagent | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C2 (10 µl) | C2 (10 µl) | C2 (10 µl) | C2 (10 µl) | C2 (10 µl) | C2 (10 µl) | C2 (10 µl) | C2 (10 µl) | C2 (100 µl) | Co (100 µl) | Co(100 µl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 | no product | no product |
| Named | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 60 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 60 |
| 65 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
Gel shows no banda at 663 bp. Instead, they show an unexpected band at ~6 Kb.
- => None of the colonies is positive.
Conoly-PCR Conditions CP.W15.B
Picked 20 colonies from plates with 10 µl and 100 µl construct 1 and construct 2 (80 colonies). 4 colonies were picked from the control (10 µl) and control (100 µl).
- In the following table construct 1 is named C1, and construct 2 = C2 and Control = Co
| Reagent | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (10 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) | C1 (100 µl) |
|---|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | A | B | C | D | E | F | G | H |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl |
| Reagent | C2 (10µl) | C2 (10µl) | C2 (10µl) | C2 (10µl) | C2 (100µl) | C2 (100µl) | C2 (100µl) | C2 (100µl) |
|---|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | I | J | K | L | M | N | O | P |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl |
| Reagent | Co (10µl) | Co (10µl) | Co (10µl) | Co (10µl) | Co(100µl) | Co(100µl) | Co(100µl) | Co(100µl) |
|---|---|---|---|---|---|---|---|---|
| Expected length [bp] | 663 | 663 | 663 | 663 | 663 | 663 | 663 | 663 |
| Named | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 |
| Primer fw 10 µM | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 | 2 µl VF2 |
| Primer rev 10 µM | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev | 2 µl Screen_delH_rev |
| Dream-Taq Polymerase (2x) | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl | 10 µl |
| ddH2O | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl | 6 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 60 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 60 |
| 65 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
- => New Gibson Assembly will be performed
Gel shows no banda at 663 bp.
- => Neither the colonies A-P show the expected band, nor the control shows any band.
Generation of DelH Plasmid 11-08
Gibson Assembly
Because of the past results, we assume that the colonies can grow because the backbone religates. We also found out that the first overhang of the backbone has some homologity with a sequence at the end and we can assume that with Gibson, it primes itself.
So in the following step we will use less backbone. We prepared an 1:10 dilution of the backbone construct.
| Reagent | Concentration [ng/µl] | Amount [µl] Mix A | Amount [µl] Mix B | Amount [µl] Mix C |
|---|---|---|---|---|
| G0 | 3.5 | 9 | 0 | 0 |
| BB | 1.8 | 1 | 1 | 1 |
| Gibson Assembly Master Mix | 2x | 10 | 10 | 0 |
The time constant for the electroporation was unexpectedly low, hopefully high enough...
 "
"