Team:Washington/LightSensing
From 2013.igem.org
(→Results) |
(→Results) |
||
| Line 255: | Line 255: | ||
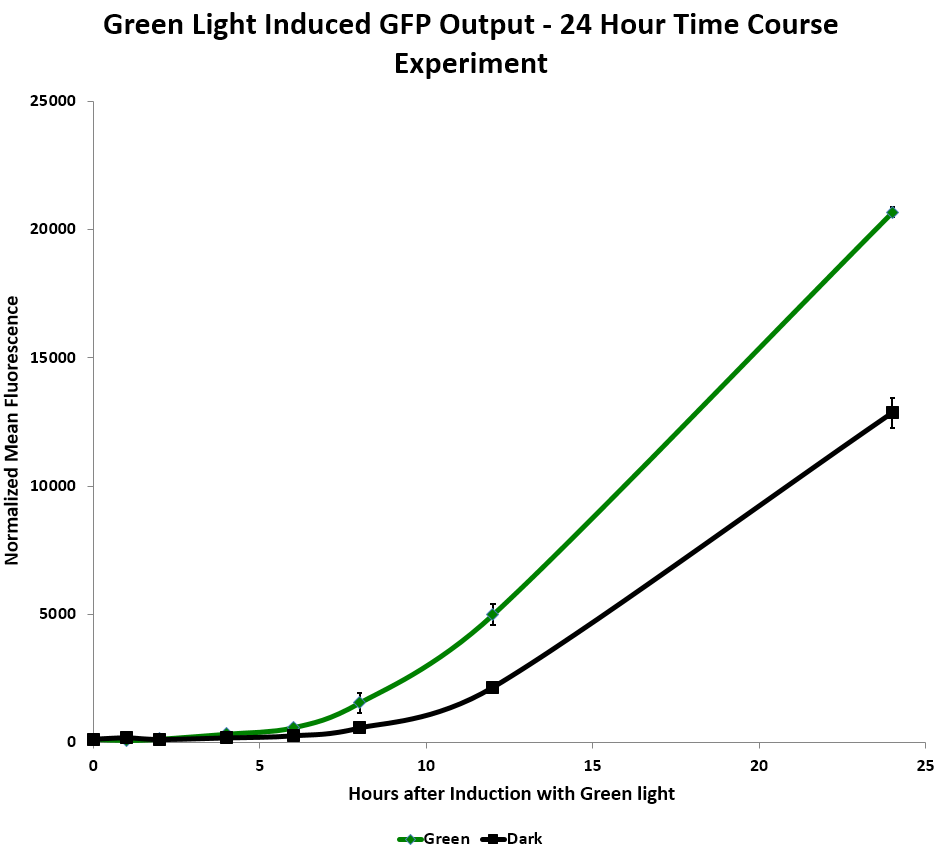
We tested relative induction levels over time by measuring the change of fluorescence over multiple time periods. When exposed to green light continuously on a shaker in liquid culture over 24 hours, GFP levels in the system increased. In the red light repressed GFP system, the red light repressed some GFP production after a few hours. However, the fluorescence increased dramatically in both dark and light systems after 12 hours, which implies that the red repressor may be leaking. (Figure 2) | We tested relative induction levels over time by measuring the change of fluorescence over multiple time periods. When exposed to green light continuously on a shaker in liquid culture over 24 hours, GFP levels in the system increased. In the red light repressed GFP system, the red light repressed some GFP production after a few hours. However, the fluorescence increased dramatically in both dark and light systems after 12 hours, which implies that the red repressor may be leaking. (Figure 2) | ||
| - | [[Image:Washington_timecourse.png|border|750px|right|thumb|Figure 2: Time | + | [[Image:Washington_timecourse.png|border|750px|right|thumb|Figure 2: GFP Output as a function of Green Light Induction Time.]] |
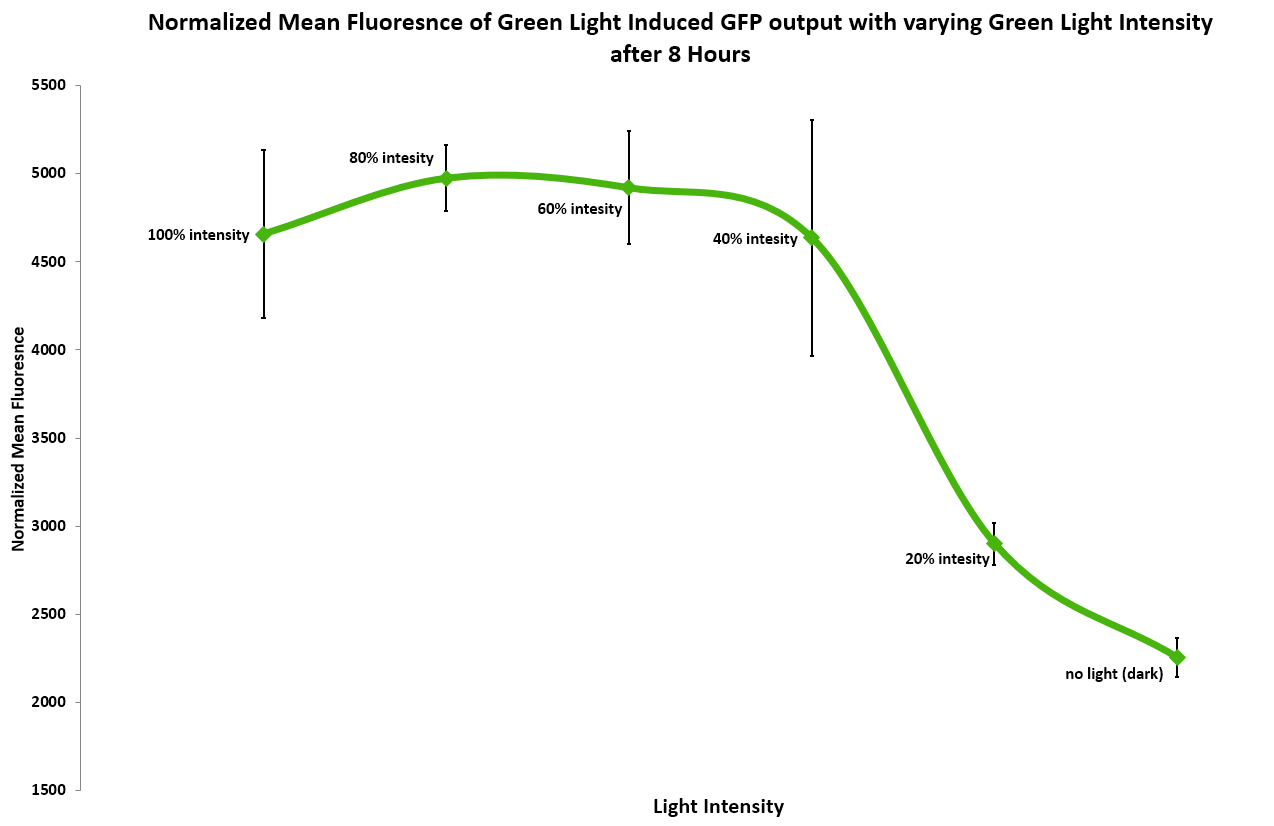
In addition, we measured the effect of light intensity on induction levels. Above 20% intensity the resulting GFP fluorescence was consistent, but it dropped at or below 20%. 40% and above gave the maximum level of induction. (Figure 3) | In addition, we measured the effect of light intensity on induction levels. Above 20% intensity the resulting GFP fluorescence was consistent, but it dropped at or below 20%. 40% and above gave the maximum level of induction. (Figure 3) | ||
| + | |||
| + | [[Image:Washington_Light_Intensity_Data.png|border|600px|light|thumb|Figure 3: Normalized Mean Fluorescence as a Function of Light Intensity.]] | ||
We tested different lengths of stopping the light between longer periods of light, with up to a second in darkness. Most of our tests in flashing light seemed to be inconclusive. (Figure 4) | We tested different lengths of stopping the light between longer periods of light, with up to a second in darkness. Most of our tests in flashing light seemed to be inconclusive. (Figure 4) | ||
Revision as of 04:40, 26 September 2013
Contents |
Background
Recently, techniques have been developed that afford researchers the ability to control gene expression with light. To date, sensors that respond to red, green, and blue wavelengths of light have been reported. Light induced expression of genes has a number of advantages over chemical induction methods. For example, light induced expression is cheaper than chemical methods, can be used to finely tune expression levels through modulations of intensity, and can be rapidly removed -- a feature lacking in chemical induction systems. Furthermore, many light expression systems are reversible, depending on the wavelength of light used. Finally, the ability to control the expression levels of multiple genes of interest simultaneously could have far reaching implications for tuning biosynthetic pathways. The use of chemical inducers for this application would likely prove to be difficult and cost prohibitive, but multichromatic gene induction represents a potential solution to this problem. To this end, the 2013 University of Washington iGEM team chose to continue a project we began in 2012 that is aimed at the development and characterization of a set of tools that bring multichromatic gene expression into the realm of possibility for synthetic biologists. In 2012, the University of Washington iGEM Team developed a freely available app for the Android operating system that affords spatiotemporal control over light wavelengths and intensities. When installed on an appropriate tablet (see E.Colight) the app could be useful for carrying out complex light induced expressions. In 2012, we were unable to fully characterize the app’s ability to control expression of light inducible genes. Thus, the 2013 iGEM Team’s goals include fully characterizing the app’s ability to control gene expression with light, and the development of a toolkit of light induced expression biobricks that are confirmed to work with the tablet app.
Our System
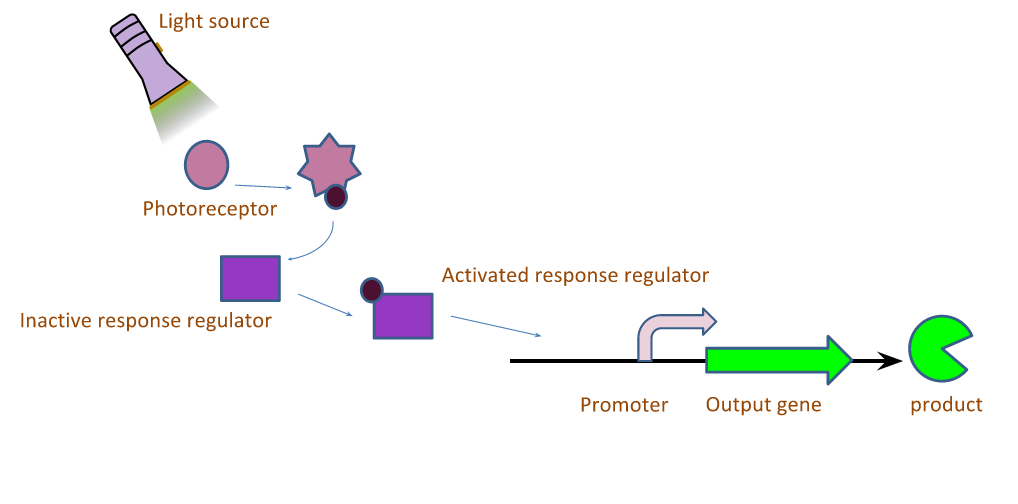
The light induced expression systems we used both require multiple protein components. In general, a small molecule chromophore is synthesized in the cell, and acts as a substrate for a transmembrane light sensing protein. When the protein sensor bound-chromophore-bound is exposed to light of the correct wavelength, a conformational change is induced in the sensor protein which in turn causes the phosphorylation of a transcription factor. The active form of the transcription factor then modulates the expression of any gene downstream of its cognate promoter. We worked with a red and green light inducible gene expression system this year. Both systems utilize the same chromophore, phycocyanobilin, but differ in the transmembrane light sensing proteins that bind the chromophore, and the downstream regulator proteins. The systems are described graphically in Figure 1.
The genes required to construct a functional light induced protein expression system were generously provided by Jeff Tabor at Rice University. Table 1.1 lists the genes required for each system and their respective functions. In order to construct a functional light responsive system, a multiple genes are transformed into E. coli. Regardless of the system in question, the minimal components required are: 1) genes utilized in the synthesis of the chromophore, 2) a transmembrane light sensing protein, and 3) response regulators which are activated by from the light sensitive proteins, and ultimately function as transcription factors.
| Source | Component | Function |
|---|---|---|
| Chromophore: | Plac/ara-HO1-PcyA | PCB chromophore expression plasmid |
| pPLPCB | Plac/ara | Hybrid lactose and arabinose promoter |
| pPLPCB | HO1 | Heme oxygenase |
| pPLPCB | PcyA | Phycocyanobillin ferridoxin oxidoreductase |
| Green light sensor: | [PCpcG2-RBS-sfGFP-CcaS](rev)-CcaR | Sense green light with GFP response |
| pJT119b | CcaS | Green light receptor |
| pJT119b | CcaR | Response regulator |
| pJT119b | PcpcG2 | Synechocystis phycocyanoybillin promoter |
| pJT119b | BBa_B0034 | Strong Elowitz RBS |
| pJT119b | sfGFP | Superfolder GFP reporter |
| Red light sensor: | Sense red light | |
| pCPH8 | PLTetO1+CPH8 | |
| pCPH8 | PLTetO1 | Tet repressible promoter constuitive in JT2 |
| pCPH8 | CPH8 | Red light receptor EnvZ fusion |
| pEO100c | POmpC-BBa_B0034-sfGFP | Red light inactivated GFP output |
| pEO100c | POmpC | OmpC promoter |
| pEO100c | BBa_B0034 | Strong Elowitz RBS |
| pEO100c | sfGFP | Superfolder GFP reporter |
| pJT106b | PLambda-RBS-LacZ-T1-POmpC-CI-T1 | Red light activated GFP output |
| pJT106b | Plambda-CI-Term | Output inverter |
Table 1: Description of source plasmids and components they contain
Our red and green light sensitive systems were constructed through transformation of a subset of the plasmids listed in Table 1. into BL21 E. coli cells. Our green light responsive system was constructed by transforming pPLPCB and pJT119b simultaneously. A red light responsive system was created by transforming pPLPCB, pCPH8, and pEO100c into E.coli. GFP was used as the output of both systems.
Methods
Cloning
Each light-responsive biosensor is composed of two or three plasmids, which requires three vectors with compatible origins of replication and orthogonal antibiotics. To subclone the light responsive operons into Biobricks, we chose equivalent copy number and antibiotic iGEM vectors. Because tetracycline is more common in iGEM vectors than spectinomycin, we chose pSB3T5 to put the chromophore operon into.Vector Equivalency Chart: https://2013.igem.org/Team:Washington/iGEMVectors
Plasmids were transformed into either XL1-blue or DH5a cells and DNA was harvested after overnight growth using a miniprep kit from Qiagen. Genes of interest were amplified using oligos with Prefix and Suffix added on to the ends, plus 3-6 extra basepairs recommended by NEB for efficient cutting. Operons from pPLPCB, pJT119b and pEO100c were amplified using oligos that compatible with insertion into the pSB1C3 backbone between Prefix and the Suffix; pJT106b was also put into pSB4A5.
Light Methods
This year our team developed a protocol optimized for the specifics of our system. Overnight cultures were grown of E. coli containing plasmids with genes for all of the parts of the light system. The next morning, these cultures were used to inoculate either M9 media or M9 agar. The app was set up for the appropriate container and light conditions, and the “dark” plate is covered in aluminum foil. These plates are placed on the tablet and grown in a 37C incubator. Cell density (absorbance) and GFP output (fluorescence) are then measured using a plate reader. We ran multiple experiments using this general protocol, including a timecourse, intensity test, and blinking test, plate image test. Timecourse experiment was done in varying amount of time points. For our plasmid, we tested them up to 16 hours. Intensity experiment was also run for our green light inducible GFP. Different percentage of intensity which could be selected in the tablet. Blinking test incorporates different timing between green and dark which could also be specified using our app. Finally we inoculate cells into media which also contains agar. Then the media + agar plates are subjected to different light conditions.
To carry out a typical Green light inducible GFP experiment:
1. Transform plasmids containing pLPCB and pJT119B operon to a competent cell. Our lab uses JT2 (Tabor, J. J. et al., Multichromatic Control of Gene Expression in Escherichia coli, J. Mol. Biol. (2010), doi:10.1016/j.jmb.2010.10.03) cells for gfp output testing.
2. Plate cells on LB agar plates with XXX and XXX antibiotics. See table below for list of systems and plasmids with their required antibiotics.
3. After incubation at 37 oC overnight, pick a single colony, and inoculate 5 mL of LB medium in a 50 mL Falcon tube.
4. Grow cultures to saturation overnight at 37 oC with shaking.
5. Determine cell density (O.D.600 measurement), and dilute overnight cultures to a final O.D.600 of 1x10-4 in M9 minimal medium supplemented with casamino acids
(link: http://openwetware.org/wiki/M9_salts 5x m9salts) (link: http://openwetware.org/wiki/Endy:M9_media/supplemented m9 supplemented)and appropriate antibiotics.
For 96 well plates:
1. Pipette 260 ul of the inoculated M9 + Casamino acid media (link: http://openwetware.org/wiki/M9_salts 5x m9salts) (link: http://openwetware.org/wiki/Endy:M9_media/supplemented into each well of interest in a clear bottomed black 96 well plate (Supplier information -- maybe even the name).
2. Cover the plate with breathable tape.
3. Orient the 96 well plate on the tablet such that the wells line up with the appropriate light source on the the tablet.
4. Secure the 96 well plate on top of the tablet with tape.
5. Incubate tablet and 96 well plate on an orbital shaker (RPM?) for 8 hours at 37 oC.
6. Remove 200 ul of culture from each well, and pipette them to 96 well plates for analysis (Highlight analysis and link to pertinent protocol).
96 well analysis (GFP expression):
1. GFP expression levels are analyzed on a spectramax m5e plate reader (bottom read) with an excitation of 480 nm, and emission at 520nm. Set the plate reader to automix the samples for 5 seconds prior to analysis.
2. GFP expression levels were normalized against cell density by dividing the relative fluorescence unit values by O.D.600 data.
For 60mm mini petridish:
1. Add 7.5ml of the inoculatted M9+cassamino acid media into the plate which is labelled for green light testing.
2. Add another 7.5ml of the inoculated media and a put it into another plate which is labelled as dark for the dark condition test.
3. Foil the mini petri dish for the dark ones.
4. Get another clear 60mm mini petri dish and then use it as a spacer plate from the tablet to the testing petri dish which will sit on top of the green light. This will prevent overheating of the culture. (refer to supplemental image)
5. Tape both spacer and testing petri dish ontop of the testing tablet securely and put them on top of the orbital shaker in the 37C incubator.
6. After eight hours, take 200ul of the culture which has been growing on top of the shaker and pipette them to 96well plates for RFU plate reading
7. We took the Optical density of the cells with lambda 600nm
8. Also took endpoint fluorescence which is excitation= 480nm, emission= 520nm, autocutoff= 515nm. Automixed 5seconds before reading.
9. Then we normalized RFU(relative fluorescence unit) with the optical density by dividing the RFU with the OD.
Results
We tested relative induction levels over time by measuring the change of fluorescence over multiple time periods. When exposed to green light continuously on a shaker in liquid culture over 24 hours, GFP levels in the system increased. In the red light repressed GFP system, the red light repressed some GFP production after a few hours. However, the fluorescence increased dramatically in both dark and light systems after 12 hours, which implies that the red repressor may be leaking. (Figure 2)
In addition, we measured the effect of light intensity on induction levels. Above 20% intensity the resulting GFP fluorescence was consistent, but it dropped at or below 20%. 40% and above gave the maximum level of induction. (Figure 3)
We tested different lengths of stopping the light between longer periods of light, with up to a second in darkness. Most of our tests in flashing light seemed to be inconclusive. (Figure 4)
We cloned CcaS, CcaR and sfGFP from pJT119b, and the chromophore producing genes XXX and XXX from pLPCB. These parts were then tested using the standard GFP output protocol against the verified light system plasmids. Green light induced expression of GFP over the dark control, confirming the functionality of these BioBrick plasmids. However, the growth rate was relatively slower than that of the control plasmids. We hypothesize that this difference is due to the use of different antibiotic resistance genes between plasmids. (FIGURE 5)
Future Plans
The potential for the use of mobile devices to facilitate light-induced expression of proteins could have far reaching impacts for synthetic biologists, iGEM teams, for those hoping to conduct biological research with limited resources. Complex spatial patterning and even simple gradients of light intensity and color would likely be of great use to the synthetic biology community. Spatial control allows the induction of cellular patterns to simulate signalling gradients in tissue development and are useful in lithography and other pattern related technology. Expanding the development of our light-inducing app will give iGEM researchers the ability to express gene products and initiate circuits simply with light in varying intensities for a range of expression levels. The 96-well format allows for the testing of many different constructs at the same time, greatly increasing the potential experimental throughput. Our proposal of a ‘hot-swappable’ designer Biobrick will allow researchers to exchange sfGFP with any custom output desired. For example, a LacZ reporter could be used with the to create conventional ‘Coliroid’ images. Additional fluorescent outputs such as RFP and CFP could be used to create separate and distinguishable signals. This will allow us to extend testing of red and green light sensors together on the same plate. Additionally, we would test the tablet App using a blue light inducible construct for instance the LovTAP system presented in 2009 by EPF-Lausanne. Our app allows the simultaneous expression on three separate chromatic channels and is thus compatible with blue light systems. Ultimately, these three signals could be used to create trichromatic output using RFP, GFP and CFP to recreate a full color image, from black and white Coliroid to vivid color bacterial photography. Finally, we are testing the tablet for it’s capacity to be used as a portable incubator and expression system based on it's heat output. Because of the high cost of purchasing a new incubator, we foresee this will be a boon to any iGEM team just starting out and will allow more teams to enter the competition and synthetic biology more accessible to high school programs. It also reduces the space requirements to run both educational and high-throughput expression experiments, as well as could be utilized in resource-limited settings for example to induce and record data from biosensors in remote locations. The app can be set up easily owing to the fact that many people already own a mobile tablet device and can turn it into an incubator by simply downloading it from the Google App Store.
Parts Submitted
This section serves as a quick lookup table for parts submitted for the 2013 Jamboree.
<groupparts>iGEM013 Washington</groupparts>
We also have an automatically generated Team Parts page.
Scientific Sources
J. J. Tabor, A. Levskaya, and C. A. Voigt, “Multichromatic control of gene expression in Escherichia coli,” J. Mol. Biol., vol. 405, no. 2, pp. 315–324, Jan. 2011. C. Voigt, Synthetic Biology, Part A: Methods for Part/Device Characterization and Chassis Engineering. Academic Press, 2011.
 "
"