Team:NYMU-Taipei/Project/Inhibition/Prohibition
From 2013.igem.org
JosephHuang (Talk | contribs) |
JosephHuang (Talk | contribs) |
||
| Line 10: | Line 10: | ||
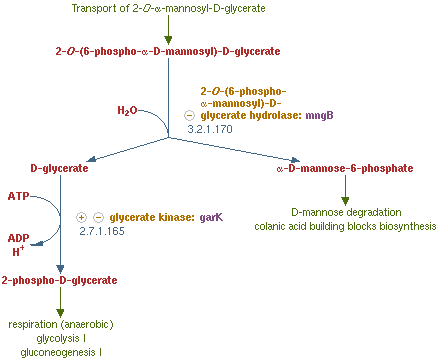
[[File:NYMU_Taipei_MngB pathway.PNG|center|thumb|325px|2-O-α-mannosyl-D-glycerate degradation pathway in MG1655]] | [[File:NYMU_Taipei_MngB pathway.PNG|center|thumb|325px|2-O-α-mannosyl-D-glycerate degradation pathway in MG1655]] | ||
MngB, a passage of gene in ''Escherichia coli'' K-12 substr MG1655, can produce alpha-mannosidase. It is an enzyme that turns 2-O-(6-phospho-α-mannosyl)-D-glycerate into mannose-6-phosphate and glycerate, which is the reverse reaction of the post-translation (glycosylation) on polar filaments of Fungi. This degradation pathway is often seen in many organisms, and the reason why we choose from MG1655 is that this species of ''E-coli'' are able to live in bees' midgut. Besides MG1655 is also the bacteria that we are going to transform our whole circuit into. This decision make our experiment more easier and more certain that this enzyme can well perform in our ''Bee. coli''. . We tent to overexpress this gene in order to inhibit the production of the PTP1 in the polar filament of ''N. ceranae'', the chosen target fungi that we are eager to eliminate.<br> | MngB, a passage of gene in ''Escherichia coli'' K-12 substr MG1655, can produce alpha-mannosidase. It is an enzyme that turns 2-O-(6-phospho-α-mannosyl)-D-glycerate into mannose-6-phosphate and glycerate, which is the reverse reaction of the post-translation (glycosylation) on polar filaments of Fungi. This degradation pathway is often seen in many organisms, and the reason why we choose from MG1655 is that this species of ''E-coli'' are able to live in bees' midgut. Besides MG1655 is also the bacteria that we are going to transform our whole circuit into. This decision make our experiment more easier and more certain that this enzyme can well perform in our ''Bee. coli''. . We tent to overexpress this gene in order to inhibit the production of the PTP1 in the polar filament of ''N. ceranae'', the chosen target fungi that we are eager to eliminate.<br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 20: | Line 15: | ||
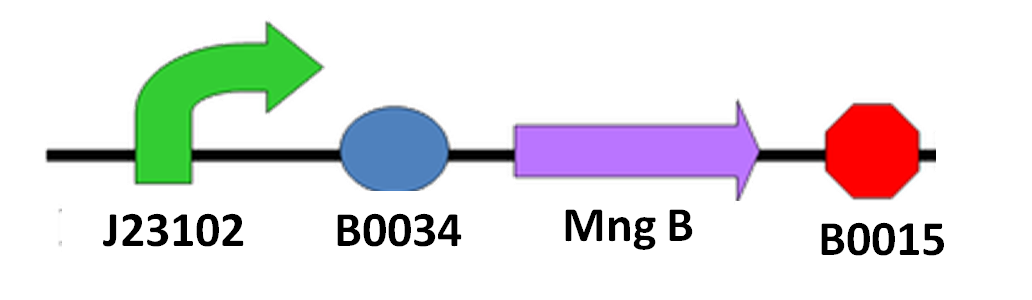
Since we cannot be sure when the spore of ''N. ceranae'' will start growing polar filament, also not having an accurate sensor to know when ''N. ceranae'' invade, we decided to add a constitutive promoter in front of mngB to let it express continuously. <br>The circuit design is as below:<br> | Since we cannot be sure when the spore of ''N. ceranae'' will start growing polar filament, also not having an accurate sensor to know when ''N. ceranae'' invade, we decided to add a constitutive promoter in front of mngB to let it express continuously. <br>The circuit design is as below:<br> | ||
[[File:NYMU_Taipei_mannosidase circuit.PNG|center|thumb|350px|circuit design]] | [[File:NYMU_Taipei_mannosidase circuit.PNG|center|thumb|350px|circuit design]] | ||
| + | |||
==Experiment== | ==Experiment== | ||
| Line 26: | Line 22: | ||
==Reference== | ==Reference== | ||
link:[http://ecocyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=EG13236-MONOMER alpha mannosidase] | link:[http://ecocyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=EG13236-MONOMER alpha mannosidase] | ||
| + | [http://www.example.com link title] | ||
{{:Team:NYMU-Taipei/Footer}} | {{:Team:NYMU-Taipei/Footer}} | ||
Revision as of 14:27, 27 September 2013


Contents |
Polar Filament Inhibition
Introduction
In order to deter the invasion of Nosema ceranae, the first step we take is to fully understand its life cycle which completes by bees. The picture underneath indicates the relation betweenNosema ceranae stage and the place it grows. By observing these information, we can obviously analyze the advantages and drawbacks at each stage, as well as decide which stage is the best and most attainable for us to weaken the transmission ability of Nosema ceranae.
Background
After N. ceranae invades epithelial cells of a bee's midgut, it will soon reproduce in two days and new generation appears. Since its lifecycle is so short that there is no time for biological methods to react and inhibit the development of the pathogen, we choose to inhibit it before entering bees' epithelial cells, which will be before N. ceranae’s germination. When N. ceranae's spore moves to bees' midgut, it will start to germinate and develop a structure called polar filament. This structure will then bind to epithelial cells of bees' midgut and poke a hole at the membrane of an epithelial cell, causing the bee eventually dead. In order to inhibit polar filament's development, we find out that there is a protein called PTP1 is highly relevant to the binding of the polar filament and epithelial cells. So we decide to suppress the growth of the polar filament by obstruct the production of PTP1. In the research that is done by other scientist, they have found out that in the process of PTP1 formation a mannose will bind to the protein. So we use mannosidase to prevent N. ceranae from producing proper PTP1 further to stop its polar filament to bind to the epithelial cell and of course, stop the infection of N. ceranae.
Mannosidase
MngB, a passage of gene in Escherichia coli K-12 substr MG1655, can produce alpha-mannosidase. It is an enzyme that turns 2-O-(6-phospho-α-mannosyl)-D-glycerate into mannose-6-phosphate and glycerate, which is the reverse reaction of the post-translation (glycosylation) on polar filaments of Fungi. This degradation pathway is often seen in many organisms, and the reason why we choose from MG1655 is that this species of E-coli are able to live in bees' midgut. Besides MG1655 is also the bacteria that we are going to transform our whole circuit into. This decision make our experiment more easier and more certain that this enzyme can well perform in our Bee. coli. . We tent to overexpress this gene in order to inhibit the production of the PTP1 in the polar filament of N. ceranae, the chosen target fungi that we are eager to eliminate.
Circuit Design
Since we cannot be sure when the spore of N. ceranae will start growing polar filament, also not having an accurate sensor to know when N. ceranae invade, we decided to add a constitutive promoter in front of mngB to let it express continuously.
The circuit design is as below:
Experiment
Reference
link:[http://ecocyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=EG13236-MONOMER alpha mannosidase] [http://www.example.com link title]
 "
"