Team:NYMU-Taipei/Project/Kill
From 2013.igem.org
(→Circuit design) |
(→Circuit design) |
||
| Line 19: | Line 19: | ||
[[image:NYMU_NO7_ethanol-producing.png]] | [[image:NYMU_NO7_ethanol-producing.png]] | ||
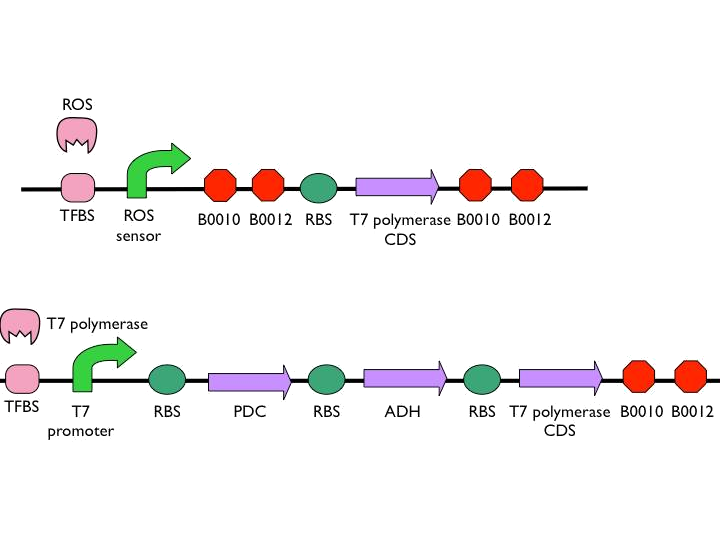
*The coding sequence of the first train is [http://parts.igem.org/Part:BBa_K145001 Part:BBa_K145001], which is the upstream DNA sequence of T7 polymerase. Otherwise, one of the coding sequence on the second strain will also be transcribed into the mRNA of T7 polymerase. In order to construct positive feedback of the alcohol-producing enzymes, we set a T7 polymerase coding sequence on the second train. Meanwhile in front of the second train, we place the receptor of T7 polymerase and [http://parts.igem.org/Part:BBa_I712074 T7 promoter]. In case of the leaky condition, we choose T7 polymerase as our go-between of alcohol-producing enzymes proliferating system. | *The coding sequence of the first train is [http://parts.igem.org/Part:BBa_K145001 Part:BBa_K145001], which is the upstream DNA sequence of T7 polymerase. Otherwise, one of the coding sequence on the second strain will also be transcribed into the mRNA of T7 polymerase. In order to construct positive feedback of the alcohol-producing enzymes, we set a T7 polymerase coding sequence on the second train. Meanwhile in front of the second train, we place the receptor of T7 polymerase and [http://parts.igem.org/Part:BBa_I712074 T7 promoter]. In case of the leaky condition, we choose T7 polymerase as our go-between of alcohol-producing enzymes proliferating system. | ||
| - | [[image: | + | [[image:NYMU_no7_circuit.png]] |
Revision as of 15:56, 27 September 2013
Contents |
Project overview
Introduction
Our aim is to resolve collapse colony disorder, which was characterized in recent years. However, it is a quite up-to-date disease, researchers have found some suspicious candidates resulting CCD. Our project focuses on one of main pathogens, Nosema ceranae. As the previous group stated, we used theoretically plausible sensor to sense Nosema ceranae, molecules to prevent Nosema ceranae from invading, and even molecules to kill Nosema ceranae. In case of uselessness of previous circuit on Nosema ceranae, we have to come up with another way to hold back the spreading of Nosema ceranae. This is what we concentrate on.
Background
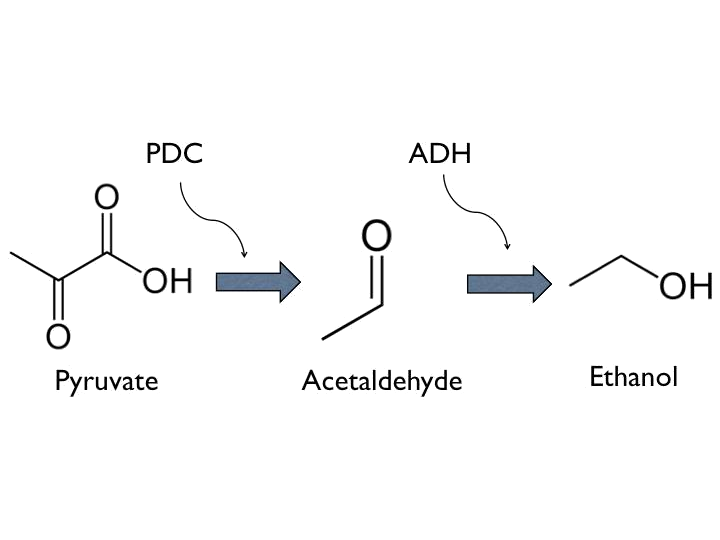
- Ethanol, is known as a kind of toxins of bees. Up to certain degree, it have potential harm to the nervous system of bees. Besides, it could have influence on hydrostatical pressure, which is one of essential factors to the germination of Nosema ceranae.
- Ethanol can be produced from pyruvate through [http://parts.igem.org/Part:BBa_K1104400 Pyruvate Decarboxylase (PDC)] and [http://parts.igem.org/Part:BBa_K1104401 Alcohol Dehydrogenase (ADH)].
Method and circuit design
Method
The infected honeybee can go back to their hive, contact other bees, and spread the pathogen in a very short time. To prevent the whole bees in hive from infected,we come up with a way to prohibit Nosema from speading. The method is composed of two parts.
- 1.How to post-sensing if Bee. coli could not successfully kill the Nosema.
We use the ROS promoter from the previous circuit. What difference between our circuit and previous circuit is we directly put two terminators after the ROS promoter to construct threshold. In case our circuit will be easily activated to cause unnecessary toll.
- 2.How to suppress the spreading of Nosema if Bee. coli failed
Inhibit the extrusion of the polar filament or let it germinate at the wrong place, and thus the spores cannot get into the intestine cell. Since the mechanism that triggers the polar filament’s extrusion is hydrostatic pressure, ethanol is our potential candidate for it is able to reduce water permeation across cell membrane and phospholipid layers, also caused a powerful inhibition of spore germination. (In this way, Nosema senses much lower hydrostatic presure).
Circuit design
- According to the result of modeling, it is obvious that we should put 1~2 terminators whose forward efficiency is about 0.98 CC after our hybrid promoter. The purpose of the terminators is to prevent bees from dying easily.The purpose of the terminators is to prevent bees from dying easily. On top of that, the other important part is the positive feedback circuit. It is useless that we only produce very few pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) to synthesize much less ethanol. Hence we introduced some biobricks to our circuit to exert efficiency.
- The coding sequence of the first train is [http://parts.igem.org/Part:BBa_K145001 Part:BBa_K145001], which is the upstream DNA sequence of T7 polymerase. Otherwise, one of the coding sequence on the second strain will also be transcribed into the mRNA of T7 polymerase. In order to construct positive feedback of the alcohol-producing enzymes, we set a T7 polymerase coding sequence on the second train. Meanwhile in front of the second train, we place the receptor of T7 polymerase and [http://parts.igem.org/Part:BBa_I712074 T7 promoter]. In case of the leaky condition, we choose T7 polymerase as our go-between of alcohol-producing enzymes proliferating system.
 "
"