Team:UChicago/Plan
From 2013.igem.org
(→Construct pUB110 BioBrick) |
(→Construct pUB110 BioBrick) |
||
| Line 32: | Line 32: | ||
In order to express kerA in B. subtilis in high amounts, we need to use a high copy number plasmid. Since high copy number plasmids for B. subtilis are not available form the iGEM HQ, we decided to design our own. We derived our design from pUB110 because it has been widely used in B. subtilis. | In order to express kerA in B. subtilis in high amounts, we need to use a high copy number plasmid. Since high copy number plasmids for B. subtilis are not available form the iGEM HQ, we decided to design our own. We derived our design from pUB110 because it has been widely used in B. subtilis. | ||
[[File:pub110.jpg]] | [[File:pub110.jpg]] | ||
| + | Source: | ||
| + | |||
| + | Boe, L. A. R. S., Gros, M. F., te Riele, H. E. I. N., Ehrlich, S. D., & Gruss, A. (1989). Replication origins of single-stranded-DNA plasmid pUB110. Journal of bacteriology, 171(6), 3366-3372. | ||
-Digest pUB110 w/ NdeI and AflII--> gel purify-->nanodrop for concentration | -Digest pUB110 w/ NdeI and AflII--> gel purify-->nanodrop for concentration | ||
Revision as of 20:45, 27 September 2013
Contents |
Summer Plan
Construct pUB110 BioBrick
In order to express kerA in B. subtilis in high amounts, we need to use a high copy number plasmid. Since high copy number plasmids for B. subtilis are not available form the iGEM HQ, we decided to design our own. We derived our design from pUB110 because it has been widely used in B. subtilis.
 Source:
Source:
Boe, L. A. R. S., Gros, M. F., te Riele, H. E. I. N., Ehrlich, S. D., & Gruss, A. (1989). Replication origins of single-stranded-DNA plasmid pUB110. Journal of bacteriology, 171(6), 3366-3372.
-Digest pUB110 w/ NdeI and AflII--> gel purify-->nanodrop for concentration
-Digest pUB110 linker w/ NdeI and AflII
Linker sequence:
attcgtcttaaggaattcgcggccgcttctagagtactagtagcggccgctgcagcatatgtcatac
-Set up overnight ligation of digested, gel purified pUB110 and digested pUB110 linker = generates pUB110 backbone
-Transform into B. subtilis to amplify
-Set up O. N. cultures
-Do B. subtilis miniprep
-Digest our pUB110 BioBrick
To test pUB110 backbone, which is kanamycin resistant, send for sequencing
-Put an upstream BioBrick (in vector with chloramphenicol resistance) + RFP BioBrick in an amp resistant backbone by doing 3 step assembly
-RFP expression from our modified pUB110 backbone would test if the backbone has all components needed for expression in B. subtilis
-Do transformation in B. subtilis
-Choose transformants--> if transformants express RFP, we could conclude our modified pUB110 backbone works--> submit pUB110 backbone to iGEM HQ
Construct kerA BioBrick
-Do Gibson assembly to put together the two kerA gBlocks from IDT
-Put our kerA biobrick into a backbone w/ amp resistance (so we can use the 3 step assembly) and transform into DH5-a
-Since promoter/upstream biobrick will be in chloramphenicol resistant vector and our puB110 vector will use kanamycin resistance, we will put the kerA biobrick in an amp resistant backbone
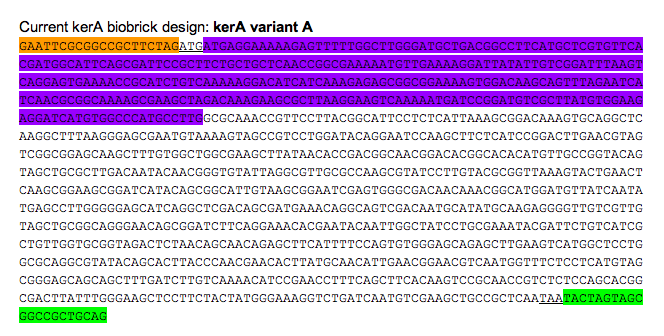
Orange: prefix
Green: suffix
Purple: kerA signal peptide
Do the following 3 step assemblies:
For E. coli BL21-De3
- Constitutive expression cassette (BBa_K314100: plate 1, well 1G chloramphenicol res vector) + KerA variant C (in amp res vector) + kan res biobrick backbone
- Lac induced expression cassette (BBa_K314103: plate 1, well 4D chloramphenicol res vector) + KerA variant C (in amp res vector) + kan res biobrick backbone
For B. subtilis
- Constitutive promoter Pveg +RBS spoVG + SacB (BBa_K541501:
plate 1, well 11E chloramphenicol res vector) + KerA variant C (in amp res vector) + kan res biobrick backbone
- Constitutive promoter Pveg + RBS spoVG (BBa_K143053: 2012 plate 3, well 20A kan and amp res vector) + KerA variant B (in chloramphenicol res vector) + kan res biobrick backbone
Pick transformants of each, do miniprep and send them for sequencing
Test keratinase expression and activity in E. coli BL21-De3 kerA transformants
Purchase commercially available keratinase A to compare activity to our keratinase Potential sources from which to purchase (since Sigma is backordered): http://www.biocat.com/products/PBL01.1.02-PR http://www.interchim.eu/reponse_cch.php?ref=520&manufacturer=0&search=keratinase&search2= http://www.interchim.fr/ft/J/JV4050.pdf http://www.proteosbiotech.com/pdfs/pure_keratinase_100_en.pdf http://www.proteosbiotech.com/index.php/en/reactivos-de-laboratorio-en/keratinasa/pure-100-keratinase-detail
Or purchase B. licheniformis PWD-1 strain.
Detect keratinase expression in cell lysate of E. coli BL21-De3 kerA transformants (constitutive and inducible) by western blot with antibody against his tag.
If expression is successful, do a cell lysis of E. coli BL21-De3 kerA transformants and test keratin azure assay.
Also, KerA could be isolated with nickel column (because kerA is his tagged).
Test keratinase expression and activity in B. subtilis kerA transformants
Grow B. subtilis kerA transformants on keratin plates (made with LB agar [nutrient rich] + keratin from DMSO dissolved feathers). Grow WT/non-transformed B. subtilis on keratin plate. Determine if there is any non-specific protease activity. If there is a clearing around the non-transformed B. subtilis controls, this is not a good way to determine keratinase expression. Compare the clearing of keratin plate accomplished by kerA transformants and WT/non-transformants. If possible, compare activity to B. licheniformis PWD-1 strain.
Grow B. subtilis kerA transformants and from a cell lysis, do western blot to detect kerA his tag expression.
After growing B. subtilis kerA transformants O. N. (unsure for how long) in liquid medium (LB broth plus antibiotic), spin down cells and store supernatant/media. Do western blot on media to detect kerA-his secretion.
Also, test keratin azure assay on supernatant from O. N. culture to determine keratinase activity.
Improve KerA activity by mutagenesis
Use Taq polymerase to introduce mutations in kerA. Transform mutated kerA into B. subtilis and plate on keratin plates. Detect clearing area around transformants. Do keratin azure assay.
 "
"