Team:Yale/Project MAGE
From 2013.igem.org
(Difference between revisions)
(→List of Papers:) |
(→Next steps) |
||
| Line 293: | Line 293: | ||
*The next step is to grow up these cultures (we sorted our 1,000 cells each time to start a culture). | *The next step is to grow up these cultures (we sorted our 1,000 cells each time to start a culture). | ||
*These cells will be tested on the plate reader compared to the wild type EcNR2, as well as the EcNR2 with our plasmid. There should be an increase in levels of fluorescence, due to increased PLA production. With only 1 MAGE cycles we were able generate a twofold increase, so in theory with an additional 5 MAGE cycles this increase will be even more drastic. | *These cells will be tested on the plate reader compared to the wild type EcNR2, as well as the EcNR2 with our plasmid. There should be an increase in levels of fluorescence, due to increased PLA production. With only 1 MAGE cycles we were able generate a twofold increase, so in theory with an additional 5 MAGE cycles this increase will be even more drastic. | ||
| - | *This experiment will be done within the next week and the results will be presented at the | + | *This experiment will be done within the next week and the results will be presented at the regional competition |
<br> | <br> | ||
<br> | <br> | ||
<br> | <br> | ||
| + | |||
== List of Papers == | == List of Papers == | ||
Jacob et al. 1997 <br> | Jacob et al. 1997 <br> | ||
Revision as of 22:47, 27 September 2013
Contents |
MAGE Targets
- The first step in applying MAGE is finding MAGE targets. This involved reading numerous scientific papers to learn as much as possible about the heterologous enzymes, and the pathway that was being used to create the PLA
Enzyme Targets
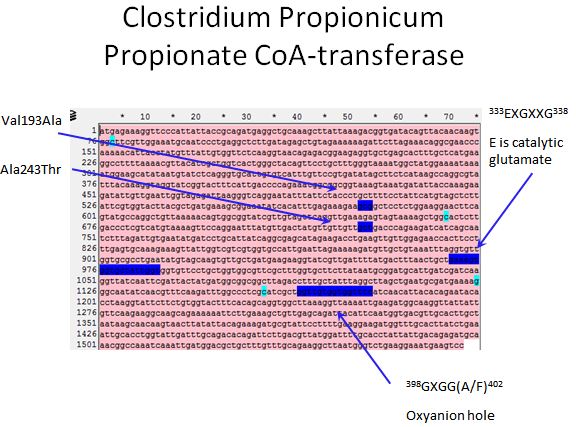
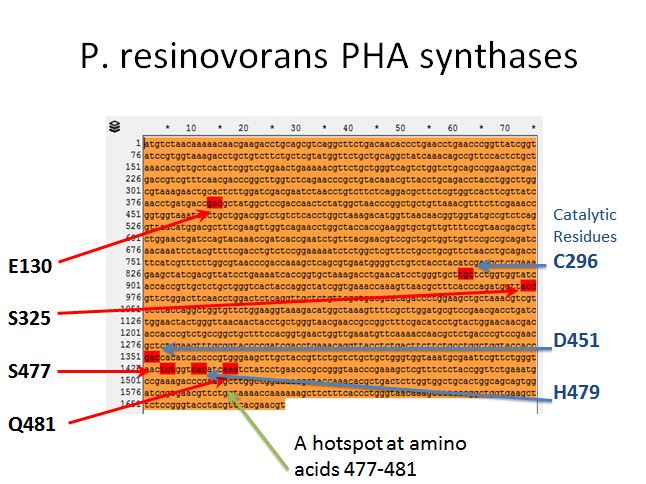
- Sadly there was no crystal structure of either enzyme we could use to locate the sites to introduce mutations
- However, we used the literature available to locate spots where we would want to introduce mutations
Propionate CoA-transferase
| 
|
P. resinovorans PHA synthases
| 
|
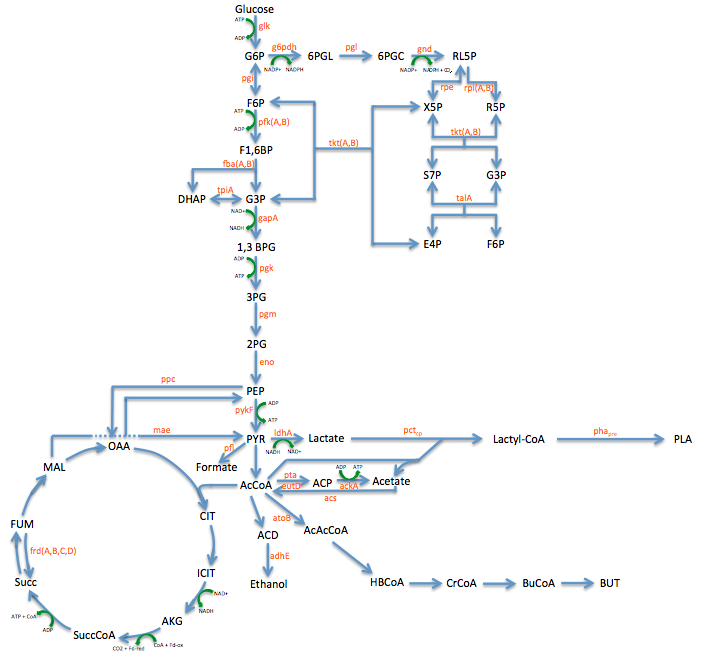
Pathway Engineering
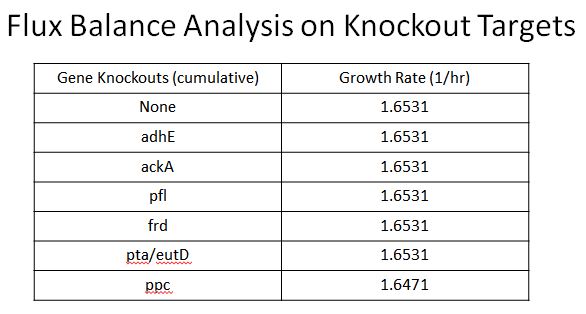
- We wanted to divert resources toward our desired pathway
- This mainly consisted of increasing the production of lactate
- In order to better understand the pathway we were tampering with we created this metabolic engineering graphic (using the sources listed at the bottom of this page)
Enzyme KOs
| 
|
RBS Tuning
| 
|
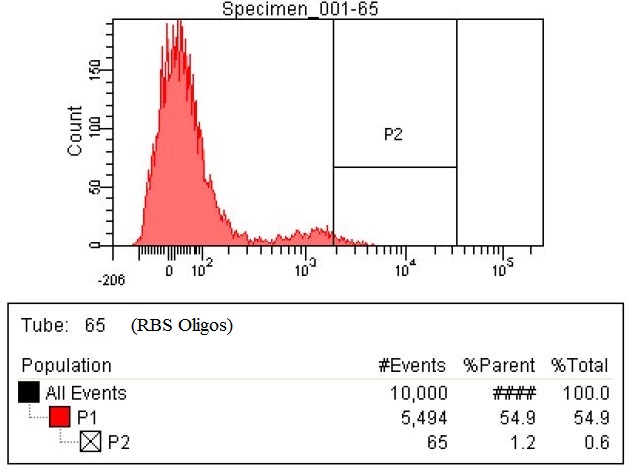
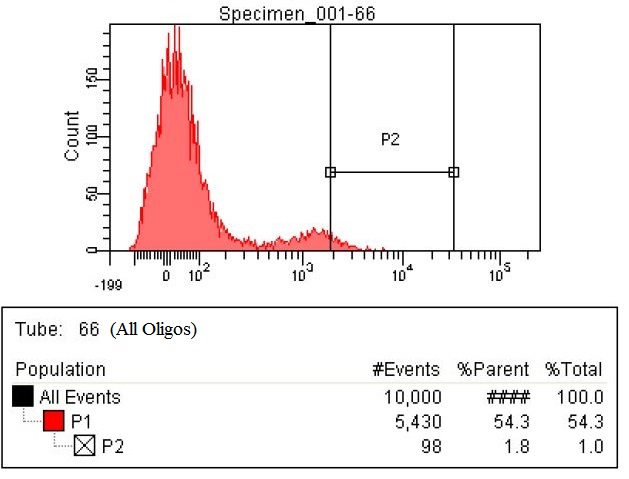
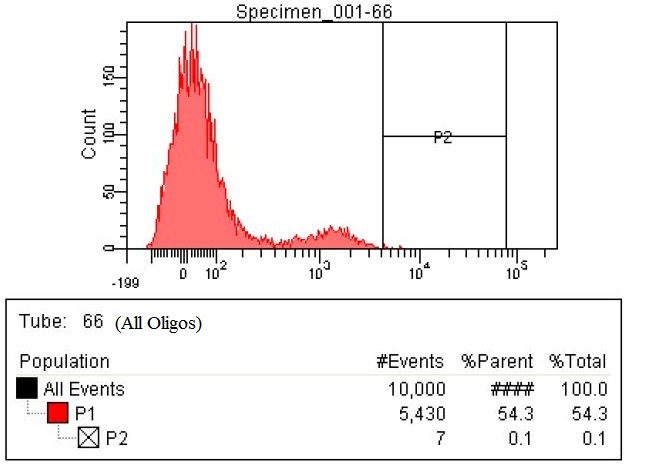
Results
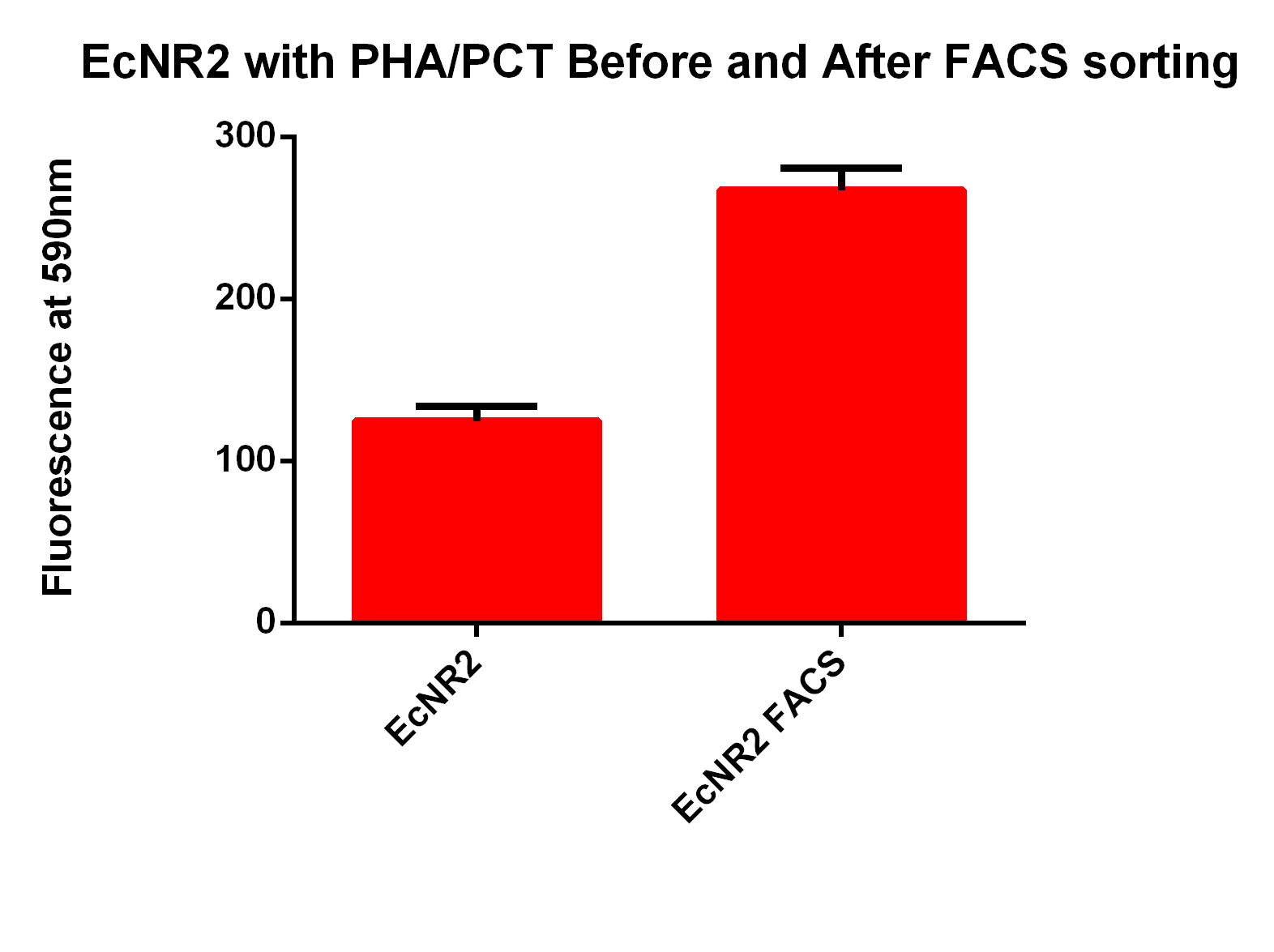
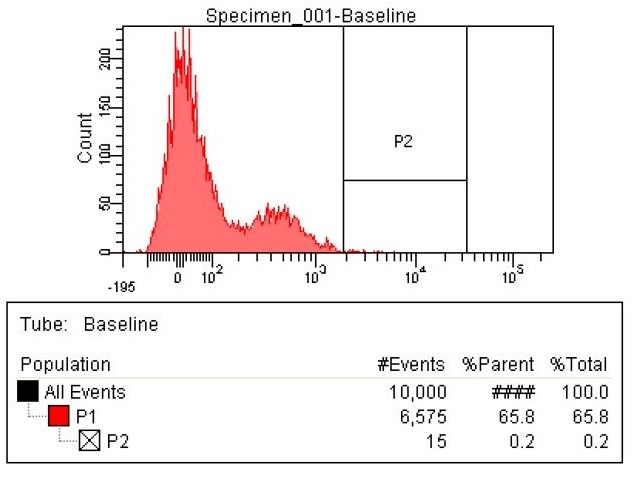
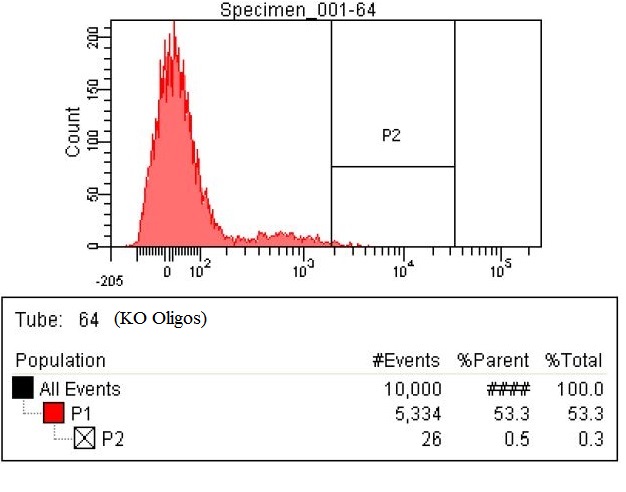
- The first time we used FACS to sort the cells, we saw roughly a two fold increase in fluorescence when we tested on the plate reader
- This was sorting after a single MAGE cycle with the KO olgios
- We used this FACS sorted strain and ran 5 more MAGE cycles (one with RBS oligos, one with KO, and one with all Oligos)
- Here are the results of the FACS sorting of these strains
Next steps
- The next step is to grow up these cultures (we sorted our 1,000 cells each time to start a culture).
- These cells will be tested on the plate reader compared to the wild type EcNR2, as well as the EcNR2 with our plasmid. There should be an increase in levels of fluorescence, due to increased PLA production. With only 1 MAGE cycles we were able generate a twofold increase, so in theory with an additional 5 MAGE cycles this increase will be even more drastic.
- This experiment will be done within the next week and the results will be presented at the regional competition
List of Papers
Jacob et al. 1997
Matsuzaki et al. 1998
Sawers et al. 1998
Park et al. 2002
Selmer et al. 2002
Takase et al. 2002
Fong et al. 2005
Matsumoto et al. 2005
Rangarajan ES et al. 2005
Matsumoto et al. 2006
Jung et al. 2009
Matsumoto et al. 2009
Juang et al. 2010
Orth et al. 2010
Yang et al. 2011
Kandasamy et al. 2012
Yang et al. 2013
 "
"