Team:SJTU-BioX-Shanghai/Prospect
From 2013.igem.org
(→Smaller Light Sensor) |
(→CRISPRi-on) |
||
| Line 28: | Line 28: | ||
<!----------------------------------------------------从这里开始写wiki---------------------------------> | <!----------------------------------------------------从这里开始写wiki---------------------------------> | ||
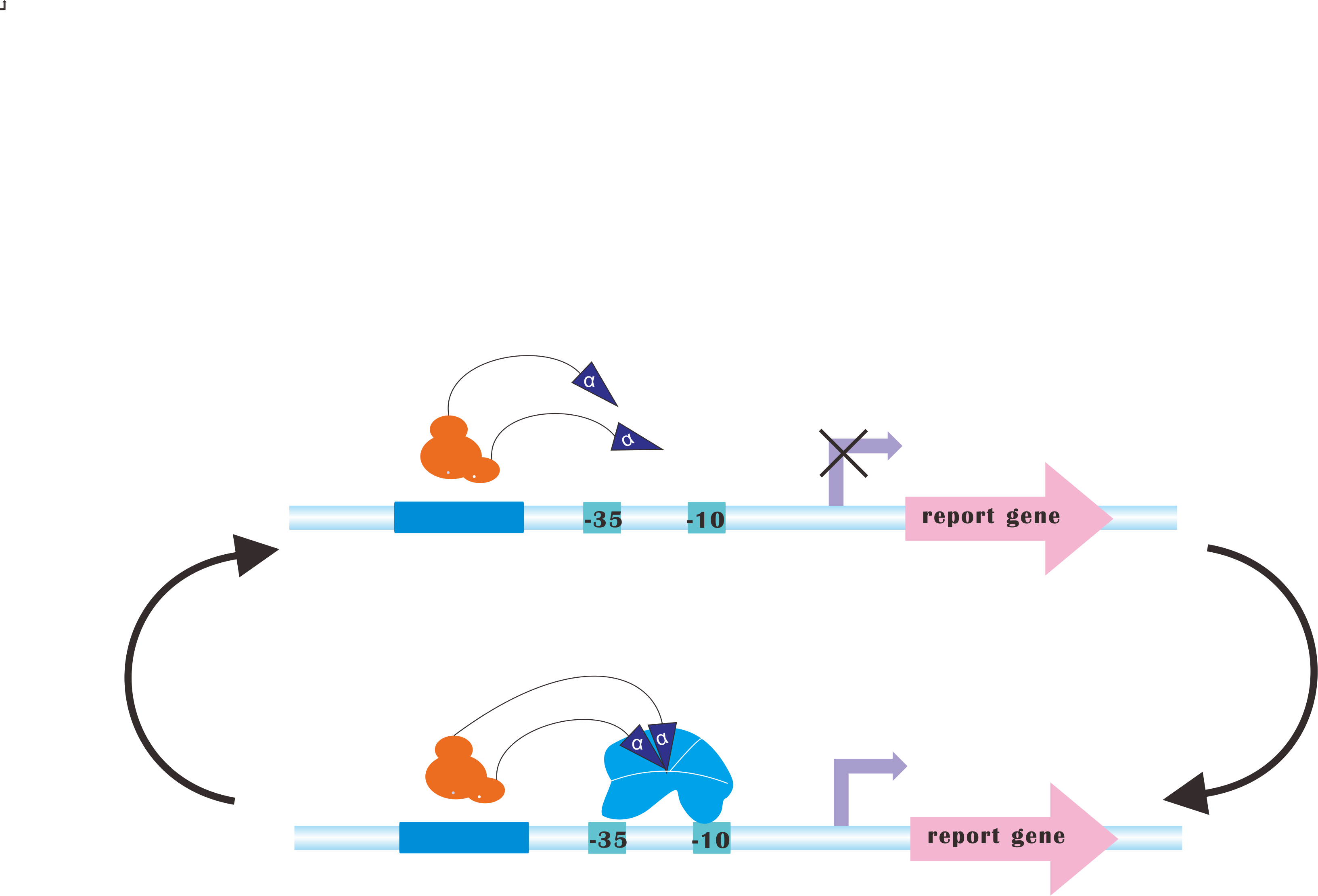
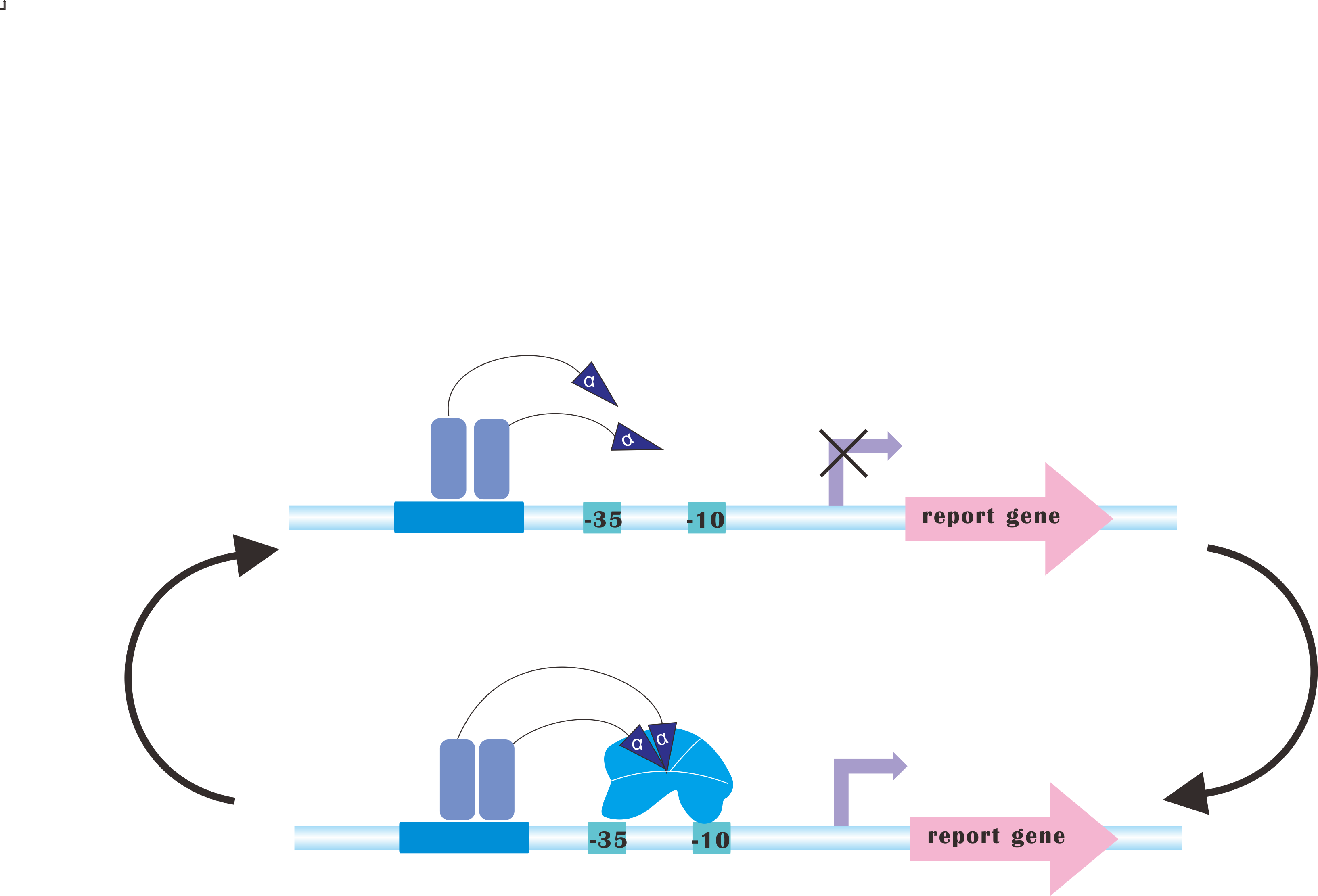
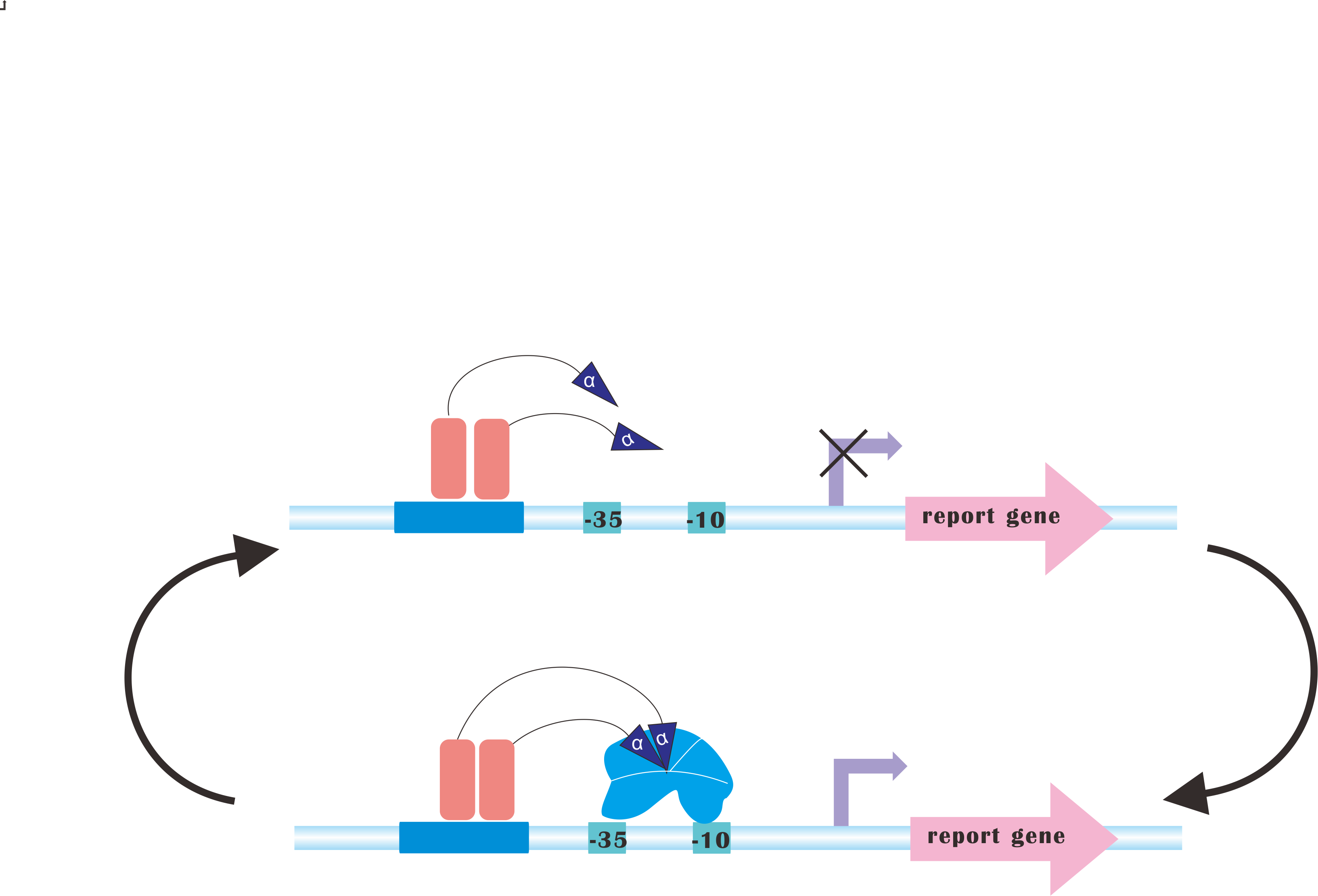
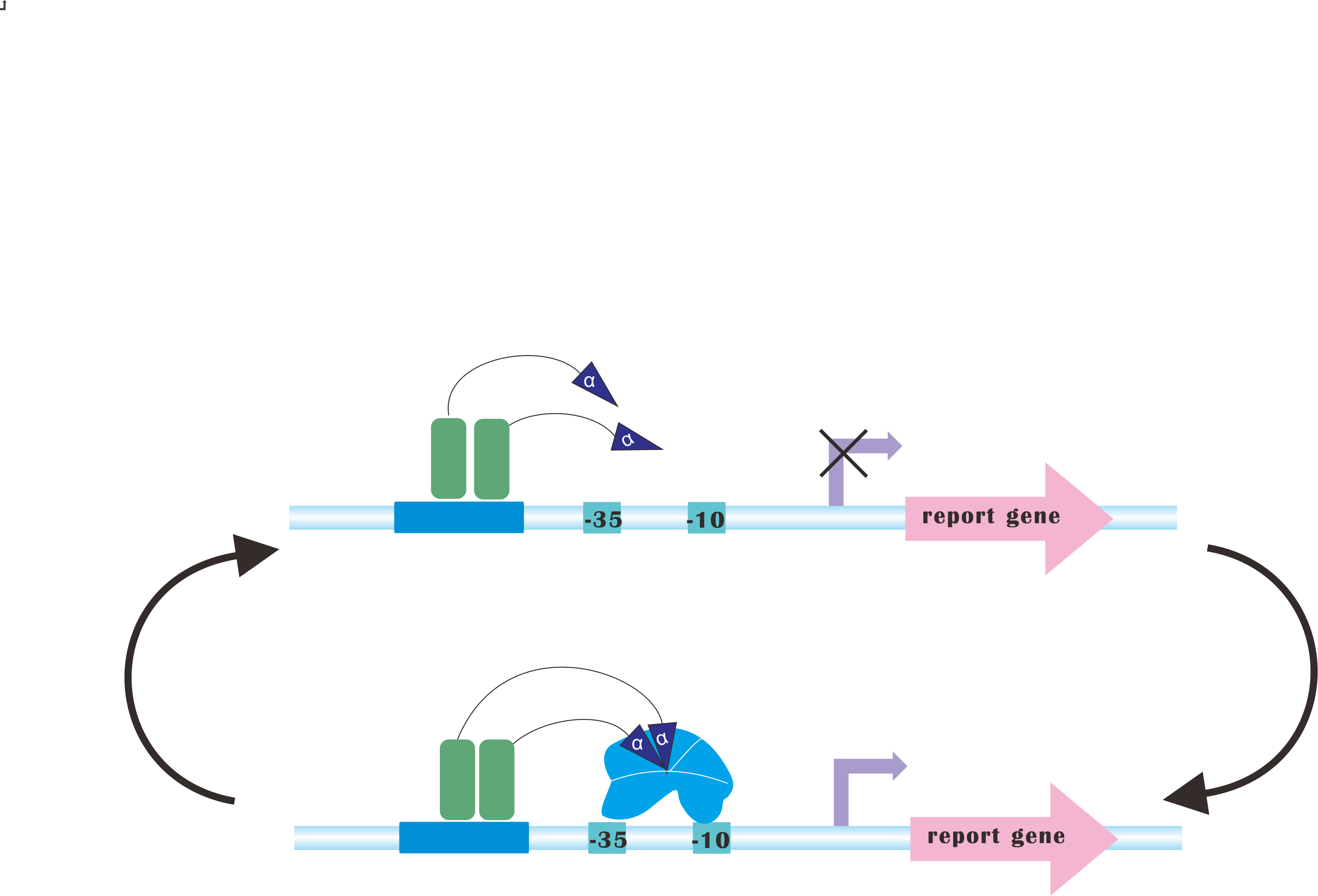
=CRISPRi-on= | =CRISPRi-on= | ||
| + | [[File:CRISPR-on.png|thumb|300px|right]] | ||
<br> | <br> | ||
At present, our Metabolic Gear Box, which combines CRISPRi with light sensors, only down-regulates three genes of a pathway. However, in many other cases, genes in a synthetic pathway are supposed to be up-regulated in order to acquire the most products. | At present, our Metabolic Gear Box, which combines CRISPRi with light sensors, only down-regulates three genes of a pathway. However, in many other cases, genes in a synthetic pathway are supposed to be up-regulated in order to acquire the most products. | ||
| Line 35: | Line 36: | ||
Our plan is to fuse alpha factors with dCas9 protein, which has previously been proved to be a successful method to create blue-light-induced transcription factors (Camsund et al., 2011). Our design is shown below. | Our plan is to fuse alpha factors with dCas9 protein, which has previously been proved to be a successful method to create blue-light-induced transcription factors (Camsund et al., 2011). Our design is shown below. | ||
<br> | <br> | ||
| - | |||
<br> | <br> | ||
However, to integrate CRISPRi and CRISPR-on would never be an easy task, since sgRNA for CRISPR-on is supposed to target upstream of promoter, rendering it necessary to incorporate logical switches. | However, to integrate CRISPRi and CRISPR-on would never be an easy task, since sgRNA for CRISPR-on is supposed to target upstream of promoter, rendering it necessary to incorporate logical switches. | ||
Revision as of 04:05, 28 September 2013
|
| ||
|
 "
"