Team:Heidelberg/Templates/Del week12 OP
From 2013.igem.org
(Difference between revisions)
FloSmi (Talk | contribs)
(Created page with "==17-07-2013== ===Amplification from FS_22 to FS_13(s); 2.7 kb=== [[File:Heidelberg_20130717 DelOP.png|150px|thumb| lane5=log2 Marker, lane6=68touchdown (FS13_short), lane7=68to...")
Newer edit →
(Created page with "==17-07-2013== ===Amplification from FS_22 to FS_13(s); 2.7 kb=== [[File:Heidelberg_20130717 DelOP.png|150px|thumb| lane5=log2 Marker, lane6=68touchdown (FS13_short), lane7=68to...")
Newer edit →
Revision as of 01:24, 30 September 2013
Contents |
17-07-2013
Amplification from FS_22 to FS_13(s); 2.7 kb
4 reactions (with long and short primer FS13 and with conditionI and conditionII)
- Reaction of DelOP
| what | µl |
|---|---|
| D.acidovorans | 1 |
| FS_22: (1/10) | 2 |
| FS_13 (long or short): (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions I of DelOP
| Biorad C1000 Touch Block B | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 1:00 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 1:00 min | |
| 1 | 72 | 5 min |
| 1 | 12 | inf |
- Conditions II of DelOP
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 1:00 min | |
| 1 | 72 | 5:00 min |
| 1 | 12 | inf |
Results:
- Amplification of DelOP failed again
- PCR will be repeated, annealing will be carried out 65°C (touchdown) as primer binding seems not to occure at high temperatues as the ones tested in the last amplification attempts
19-07-2013
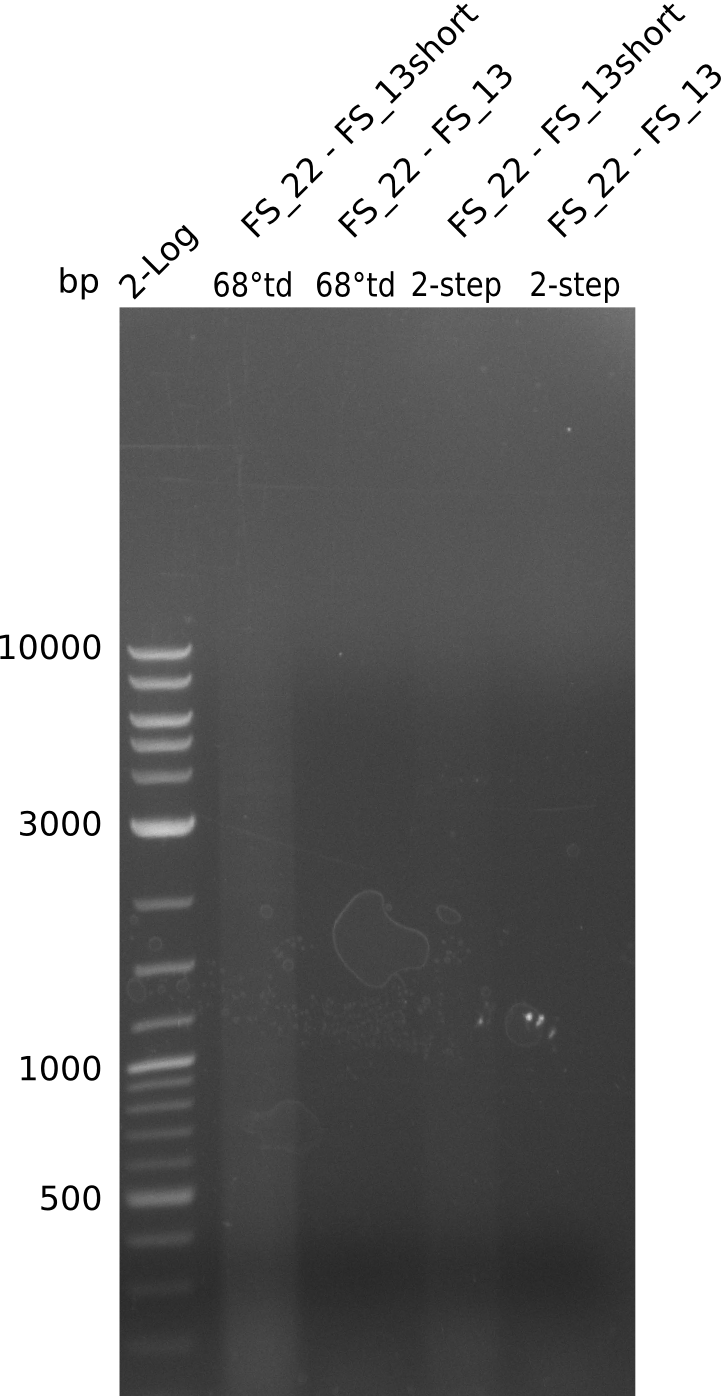
Amplification from FS_22 to FS_13s; 2.7 kb
--> reaction mixture with and without DMSO
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1/- |
| dd H2O | 4/5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
- Amplification of DelOP failed again, unexpected bands as well as a smear occured
- Annealing temperature will be further decreased, to investigate if amplification of the intended product occurs at lower temperatures
20-07-2013
Amplification from FS_22 to FS_13s; 2.7 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 4 |
- Conditions
| Biorad C1000 Touch Block B | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 62 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 60 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
- Amplification of DelOP did not work, only unintented products as well as a slight smear occured
- Reaction will be carried out again at lower annealing temperature to allow primer binding to the intended sequences
21-07-2013
Amplification from FS_22 to FS_13s; 2.7 kb
4x 20µl (with, without DMSO; 60touchdown, 72 twostep)
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1/- |
| dd H2O | 4/5 |
- Conditions I
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 60 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 58 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
- Conditions II
| Biorad MyCycler | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 72 | 1:00 min | |
| 1 | 72 | 5:00 min |
| 1 | 12 | inf |
Results:
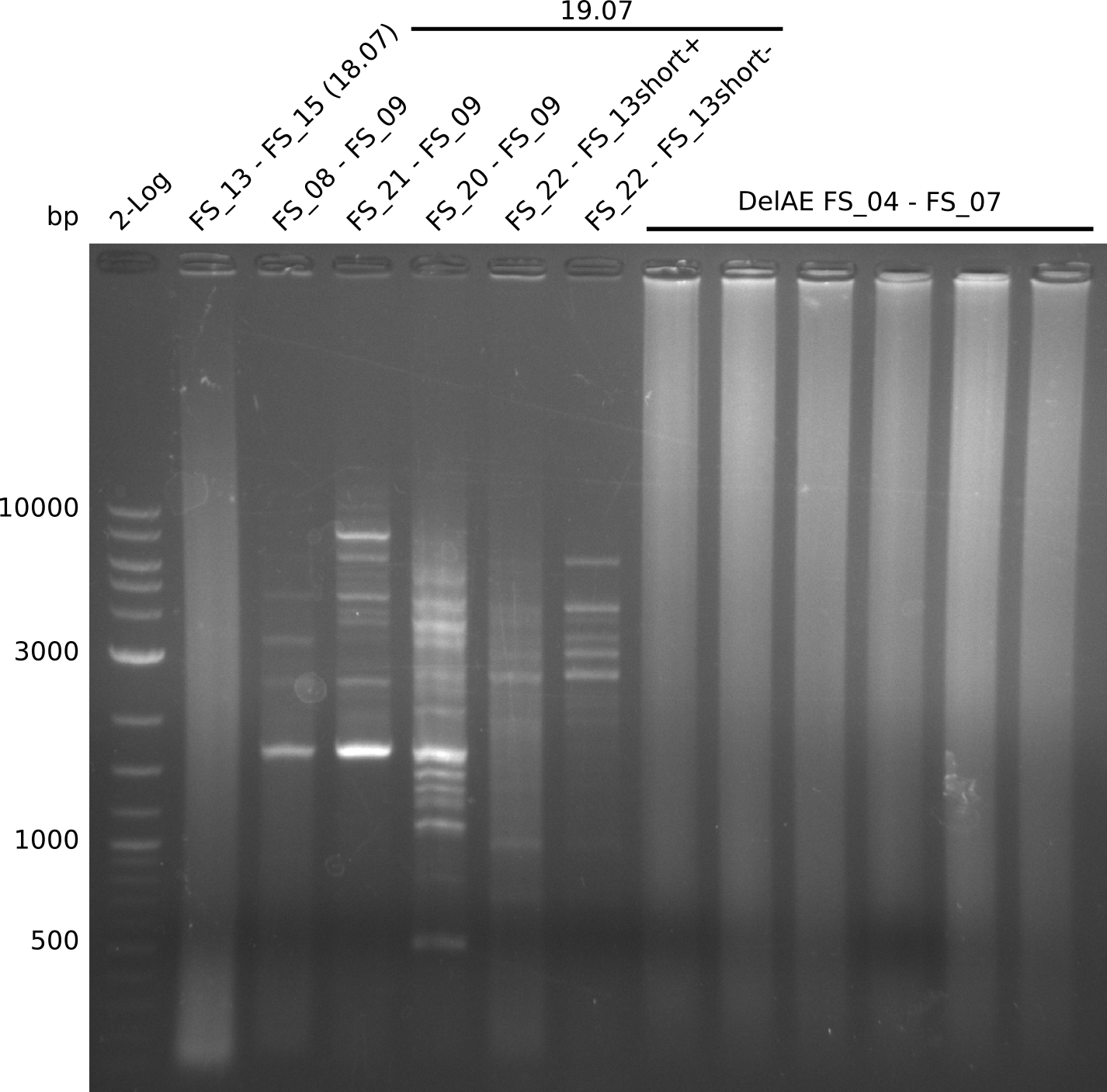
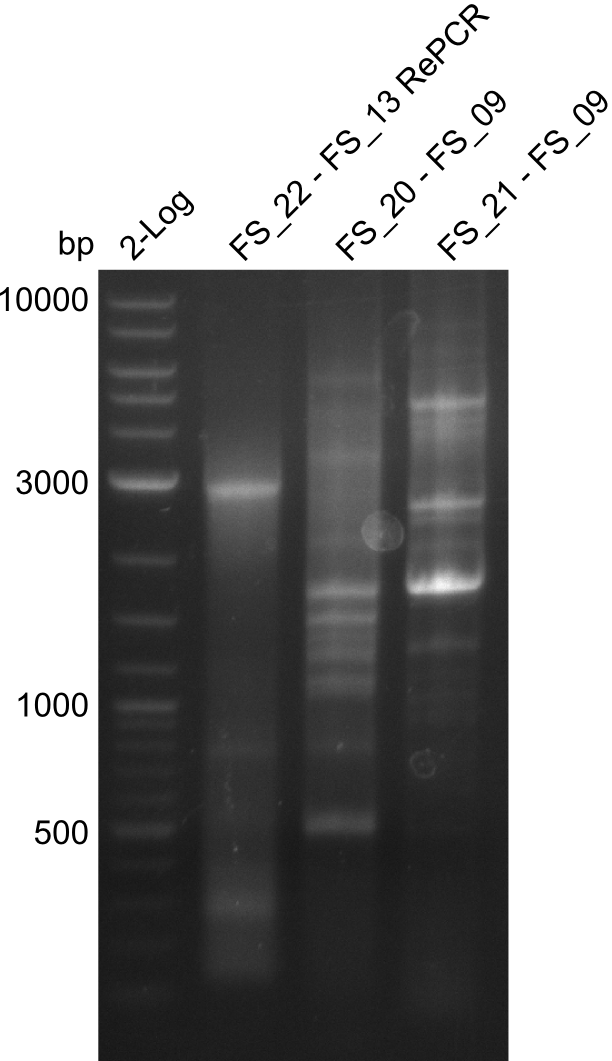
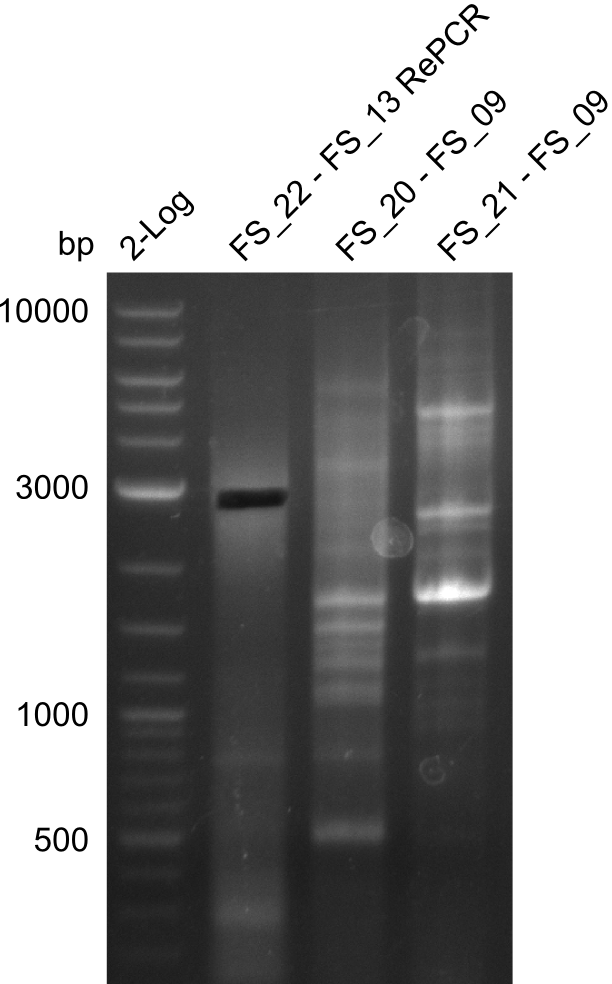
- Amplification of DelOP led to inconclusive results, a very thin band of the intended length as well as several other bands and a smear occured
- Band were cut out and DNA purified using QIAquick Gel Extraction Kit to verify amplicon in a re-PCR
Re-PCR of DelOP (FS_22 to FS_13s; 2.7 kb; 19-07-2013)
- Reaction
| what | µl |
|---|---|
| Fragment FS_22 to FS_13_short (19-07-2013) | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | - |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
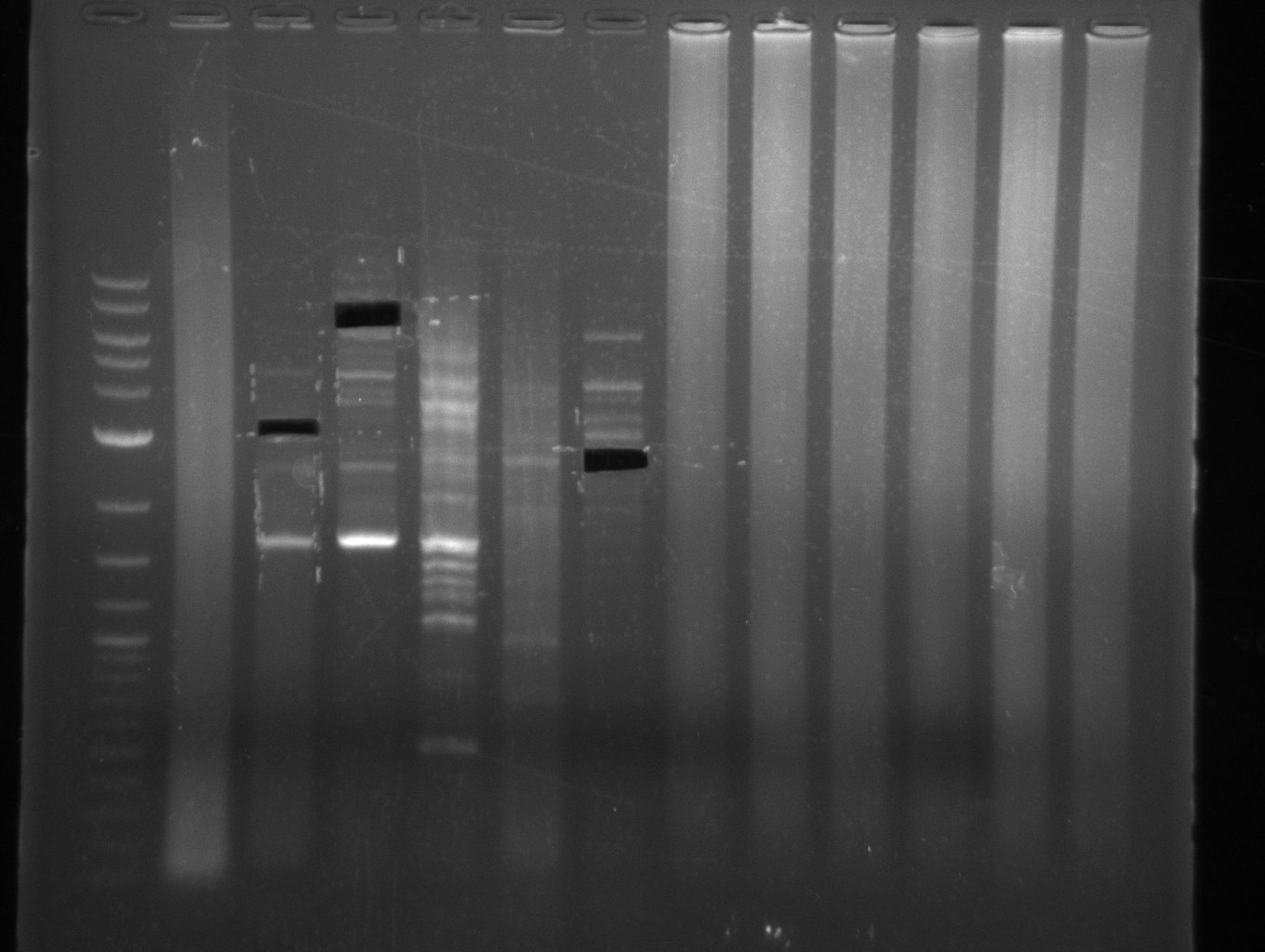
- Amplification of DelOP did not work
- PCR will be repeated as re-pcr from the previously obtained sample
22-07-2013
Re-PCR of DelOP (FS_22 to FS_13; 2.7 kb; 19-07-2013)
- Reaction
| what | µl |
|---|---|
| Fragment FS_22 to FS_13_short 19-07-2013) | 1 |
| FS_22: (1/10) | 2 |
| FS_13_long: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
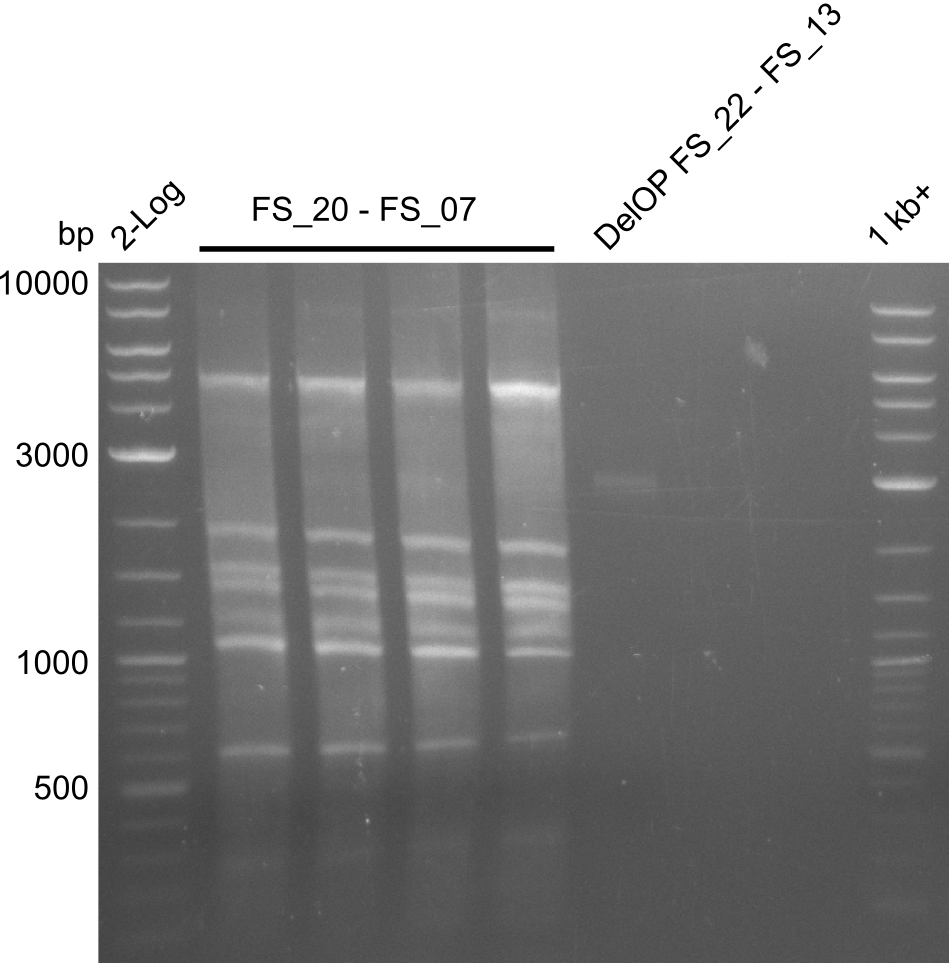
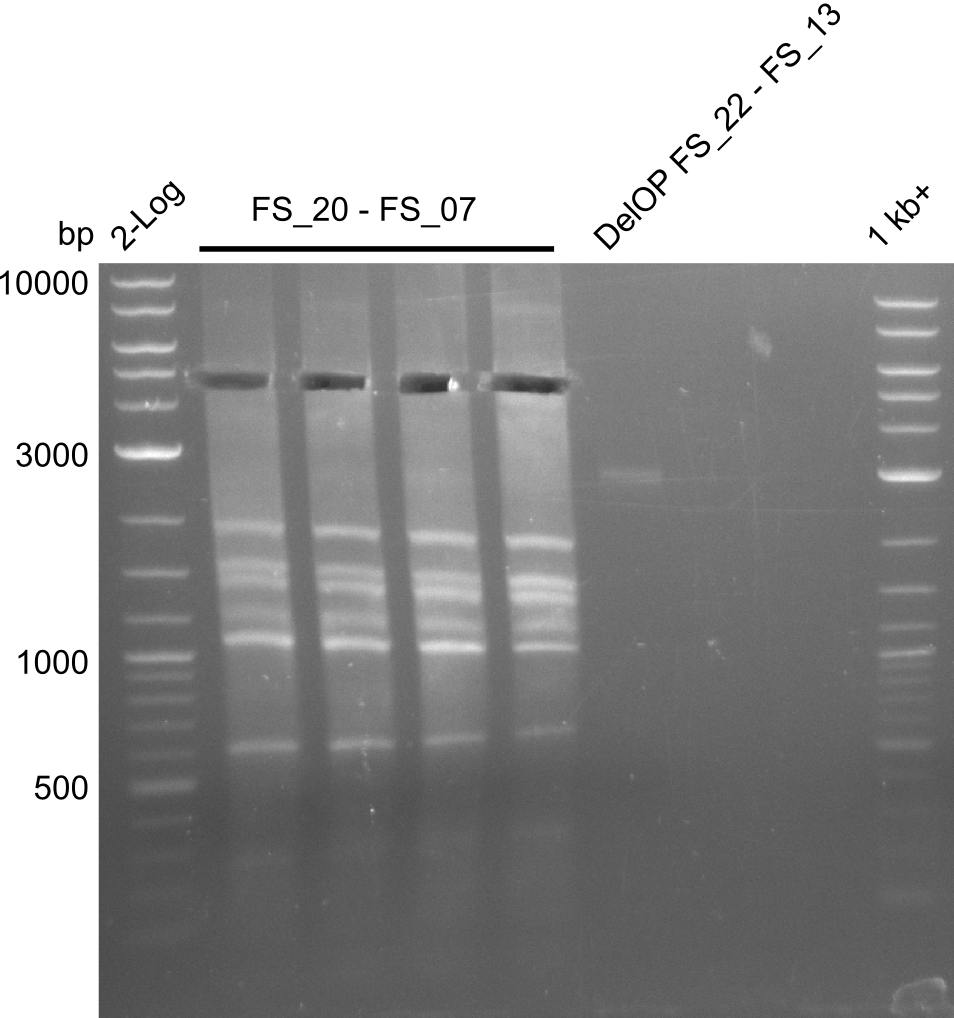
- Re-PCR did lead ot a band at the expected size but also a smear and several unexpected bands
- Re-PCR will be repeated
24-07-2013
Re-PCR of DelOP (FS_22 to FS_13; 2.7 kb; 19-07-2013)
3x20µl
- Reaction
| what | µl |
|---|---|
| Fragment FS_22 to FS_13_short (19-07-2013) | 1 |
| FS_22: (1/10) | 2 |
| FS_13_long: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
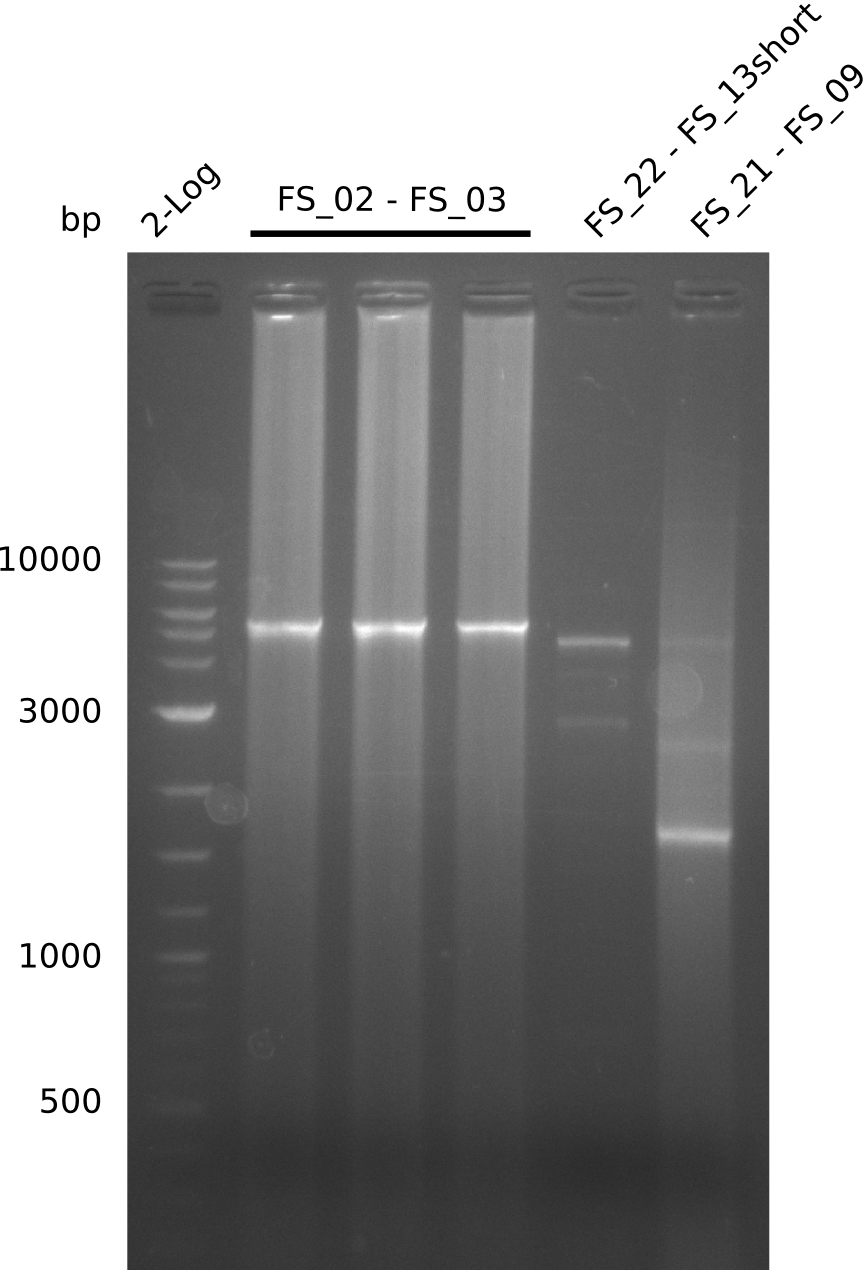
- Re-PCR of DelOP did not work, no bands were visible
- Re-PCR will be repeated
 "
"