Team:Heidelberg/Templates/Del week14 OP
From 2013.igem.org
(Difference between revisions)
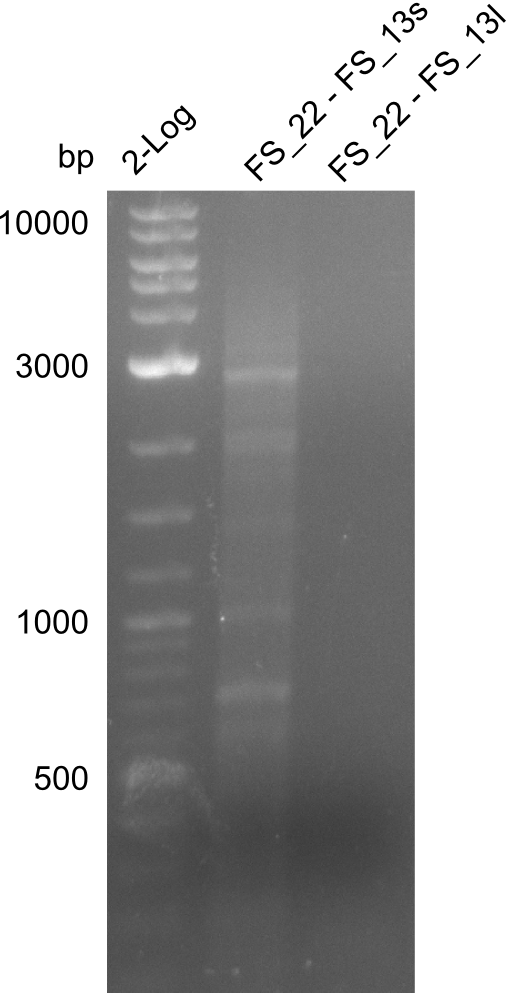
(Created page with "==01-08-2013== ===Amplification from FS_22 to FS_13(s); 2.7 kb=== [[File:Heidelberg_20130802 log2 OPsslow OPlslowbesch.png|150px|thumb|Amplification of DelOP(FS22 to FS13short/l...") |
|||
| Line 1: | Line 1: | ||
| + | ==30-07-2013== | ||
| + | ===Re-PCR of DelOP FS_22 to FS_13; 2.7 kb; [[DelO-P#19-07-2013|19-07-2013]])=== | ||
| + | |||
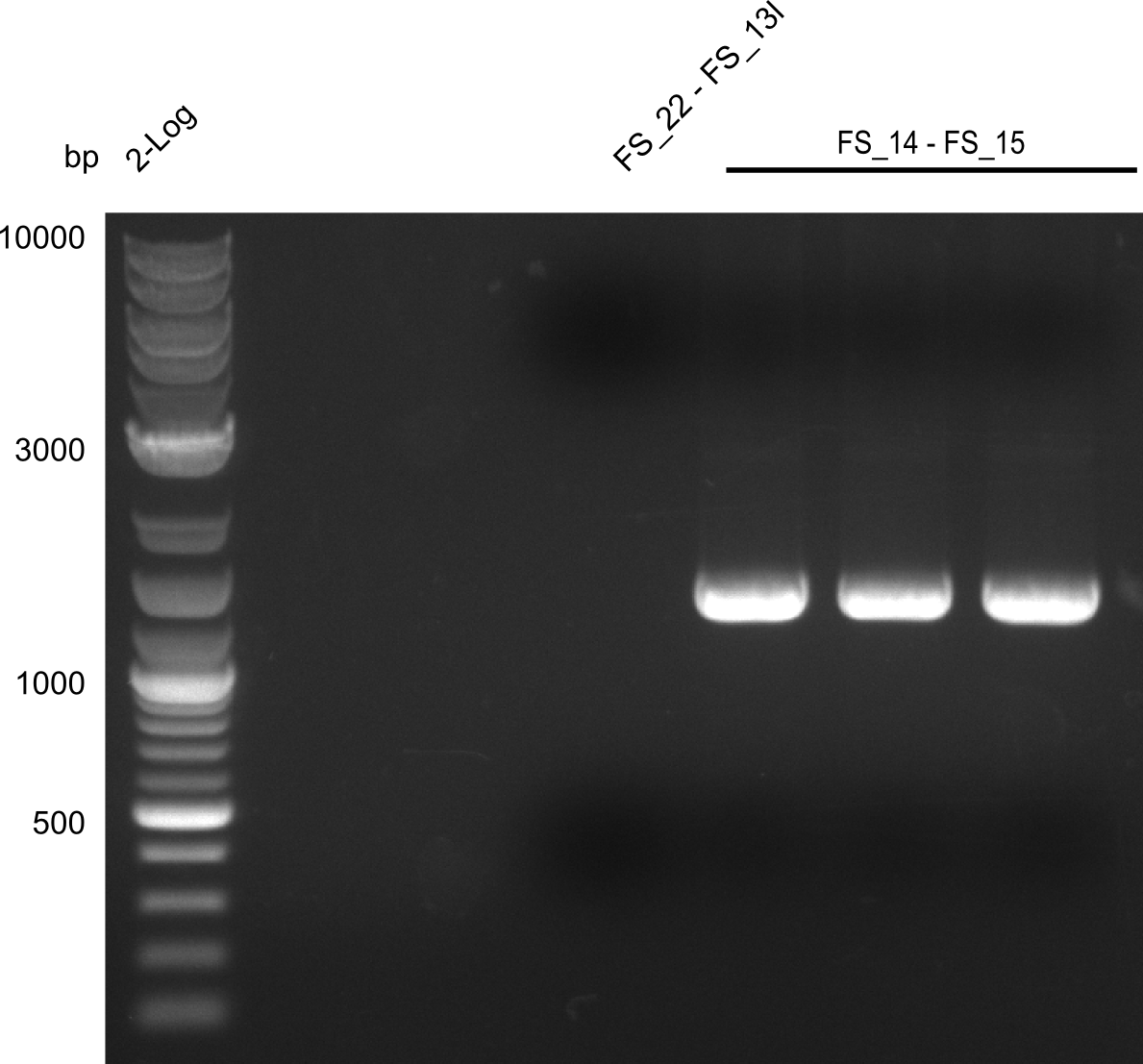
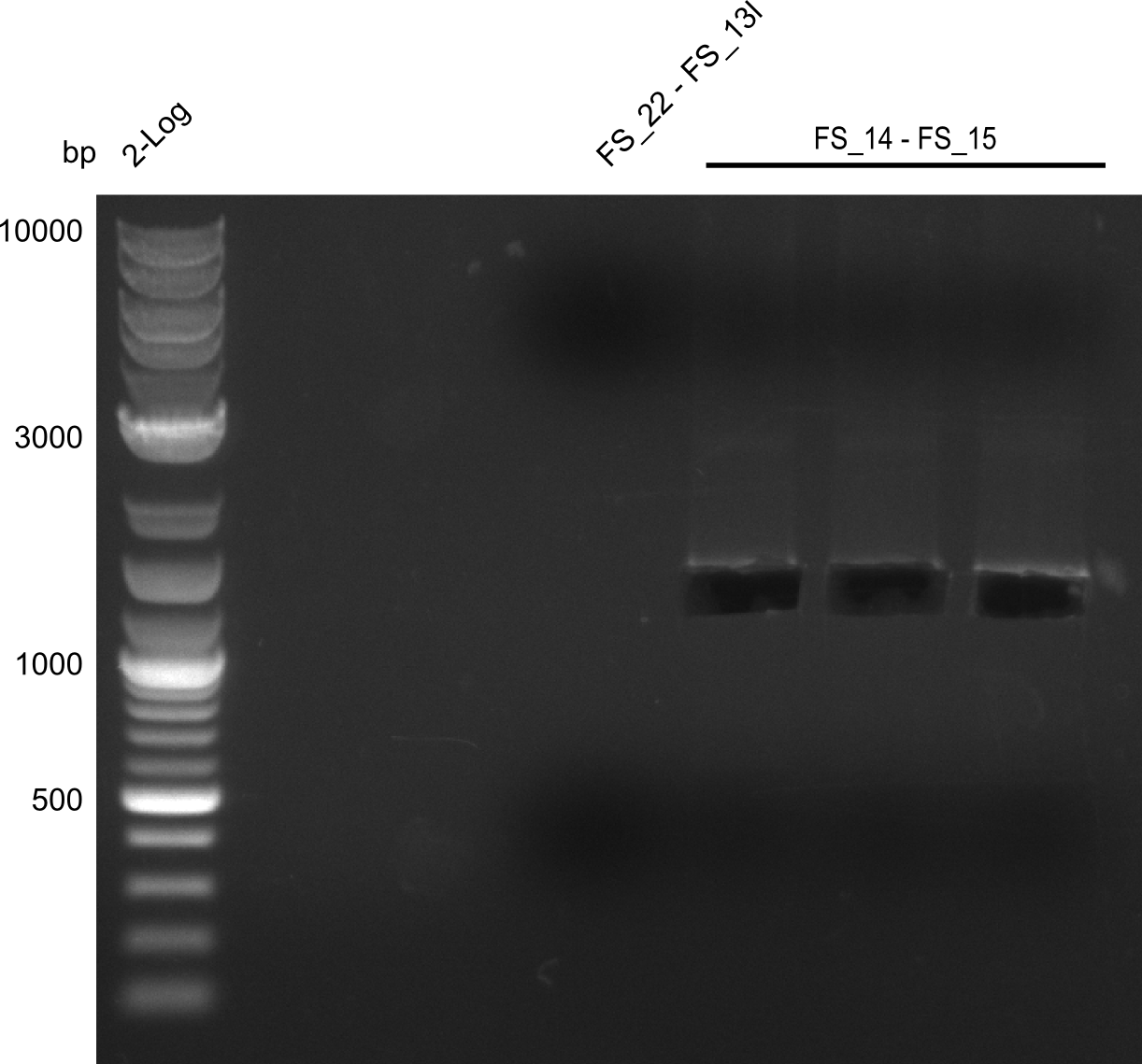
| + | [[File:Heidelberg_20130730 3x 22-13long opecoR1 log2 Tyr 3 13 15besch.png|150px|thumb|Re-PCR OP, Restriction digest OP with EcoRI; run at 100 V, 0.8 % gel (TAE)]] | ||
| + | |||
| + | 3x20µl | ||
| + | :'''Reaction''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! what !! µl | ||
| + | |- | ||
| + | | Fragment FS_22 to FS_13_short ([[DelO-P#19-07-2013|19-07-2013]]) || 1 | ||
| + | |- | ||
| + | | FS_22: (1/10) || 2 | ||
| + | |- | ||
| + | | FS_13_long: (1/10) || 2 | ||
| + | |- | ||
| + | | Phusion flash Master Mix || 10 | ||
| + | |- | ||
| + | | dd H<sub>2</sub>O || 5 | ||
| + | |} | ||
| + | |||
| + | :'''Conditions''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! colspan="3" | Biorad MyCycler* | ||
| + | |- | ||
| + | ! Cycles !! temperature [°C] !! Time [s] | ||
| + | |- | ||
| + | | 1 || 98 || 10 | ||
| + | |- | ||
| + | | rowspan="3"| 12 || 98 || 1 | ||
| + | |- | ||
| + | | 65 ↓ 0.5 || 5 | ||
| + | |- | ||
| + | | 72 || 1:00 | ||
| + | |- | ||
| + | | rowspan="3"| 18 || 98 || 1 | ||
| + | |- | ||
| + | | 63 || 5 | ||
| + | |- | ||
| + | | 72 || 1:00 | ||
| + | |- | ||
| + | | 1 || 72 || 5min | ||
| + | |- | ||
| + | | 1 || 12 || inf | ||
| + | |} | ||
| + | |||
| + | '''Results:''' | ||
| + | * Amplification of DelOP did not work | ||
| + | * PCR will be repeated with different primer concentrations to estimate whether primer dimes might be the reason for insufficient amplification | ||
| + | |||
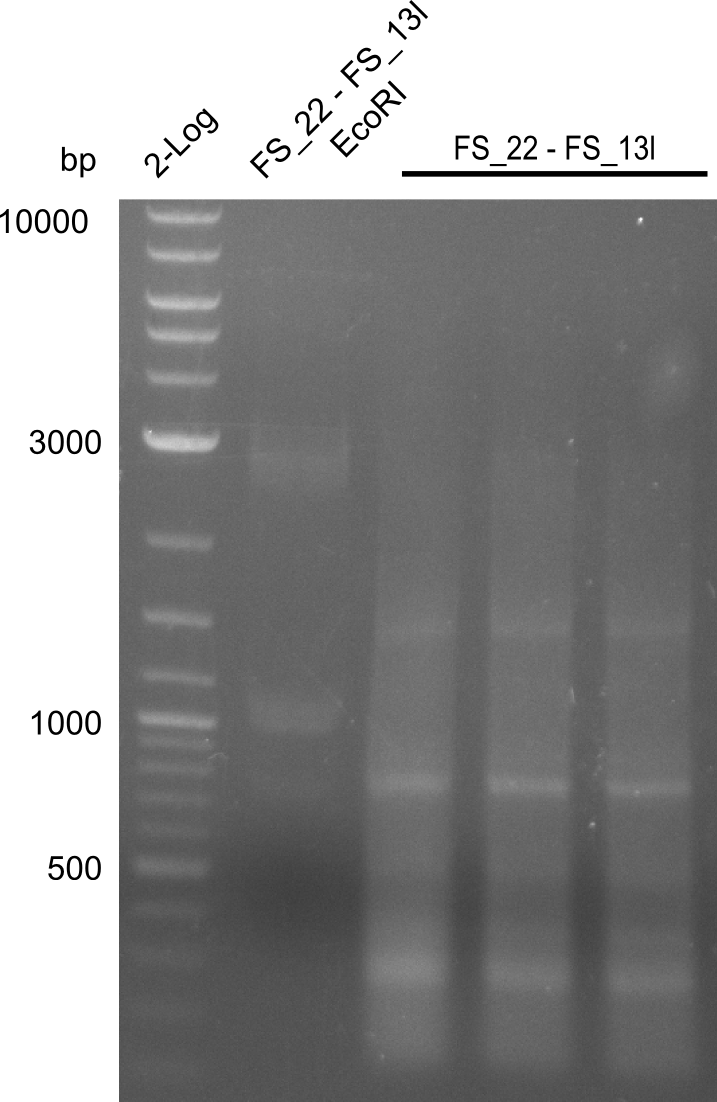
| + | ===Restriction digest of fragment FS_22 to FS_13; 2.7 kb; [[DelO-P#25-07-2013|25-07-2013]]) with EcoRI-HF=== | ||
| + | [[File:Heidelberg_20130730 3x 22-13long opecoR1 log2 Tyr 3 13 15besch.png|150px|thumb|Re-PCR OP, Restriction digest OP with EcoRI; run at 100 V, 0.8 % gel (TAE)]] | ||
| + | |||
| + | Incubation at 37°C for | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! what !! µl | ||
| + | |- | ||
| + | | FS_22 to FS_13long([[DelO-P#25-07-2013|25-07-2013]]) || 17 | ||
| + | |- | ||
| + | | EcoRI || 1 | ||
| + | |- | ||
| + | | Buffer CutSmart || 2 | ||
| + | |- | ||
| + | | Expected fragment lengths [bp] || 1883, 960 | ||
| + | |} | ||
| + | |||
| + | <div style="clear:both"></div> | ||
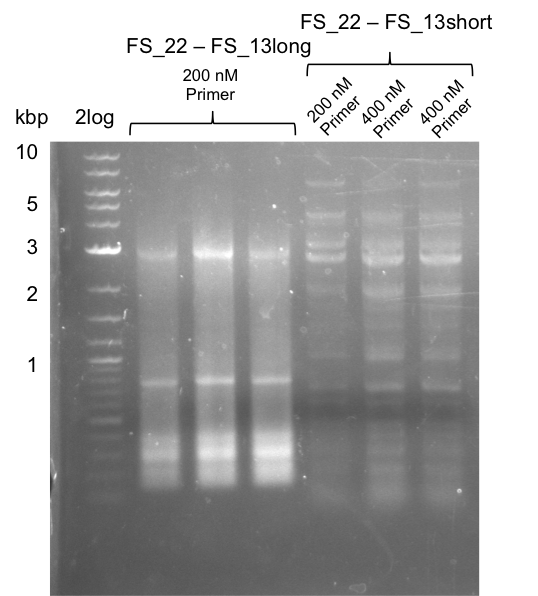
| + | ===Amplification from FS_22 to FS_13s; 2.7 kb=== | ||
| + | |||
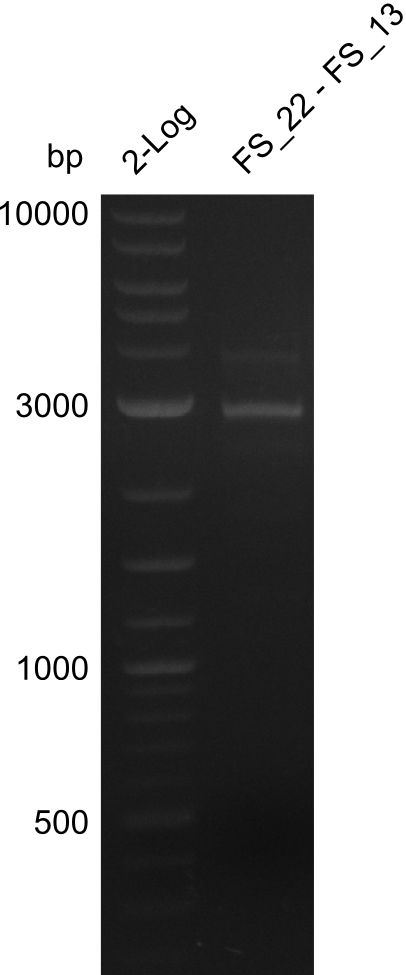
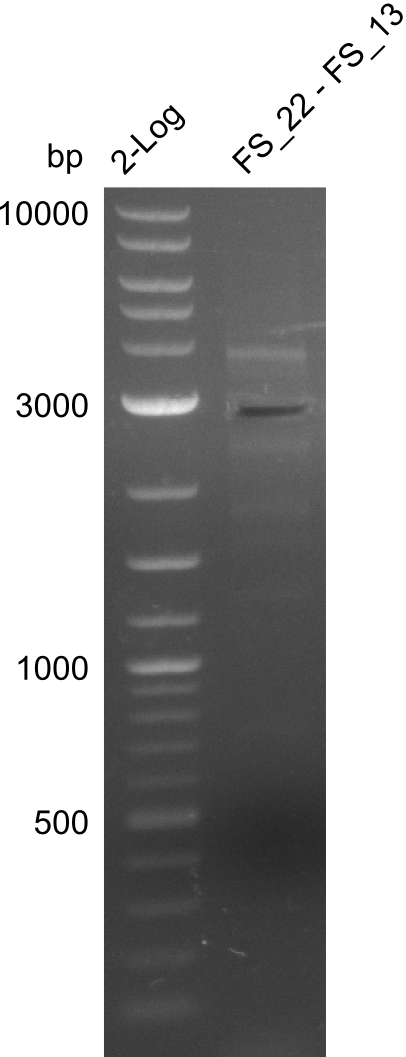
| + | [[File:Heidelberg_20130730 2log 3xre-pcr-FS22to13long FS22to13short2u-prim 2xFS22to13short4u-prim.png|300px|thumb|Amplification of Del O-P from FS_22 to FS_13long/FS_13short under different conditions run at 100 V 0.8% agarose gel (TAE]] | ||
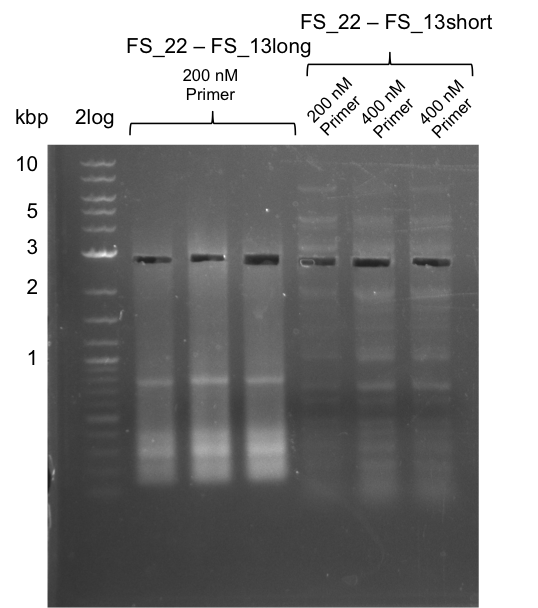
| + | [[File:Heidelberg_20130730 2log 3xre-pcr-FS22to13long FS22to13short2u-prim 2xFS22to13short4u-prim cut.png|300px|thumb|Gel after excision]] | ||
| + | |||
| + | 2x20µl | ||
| + | :'''Reaction''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! what !! µl | ||
| + | |- | ||
| + | | ''D.acidovorans'' || 1 | ||
| + | |- | ||
| + | | FS_22: (1/10) || 4 | ||
| + | |- | ||
| + | | FS_13_short: (1/10) || 4 | ||
| + | |- | ||
| + | | Phusion flash Master Mix || 10 | ||
| + | |- | ||
| + | | dd H<sub>2</sub>O || 1 | ||
| + | |} | ||
| + | |||
| + | 20µl | ||
| + | :'''Reaction''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! what !! µl | ||
| + | |- | ||
| + | | ''D.acidovorans'' || 1 | ||
| + | |- | ||
| + | | FS_22: (1/10) || 2 | ||
| + | |- | ||
| + | | FS_13_short: (1/10) || 2 | ||
| + | |- | ||
| + | | Phusion flash Master Mix || 10 | ||
| + | |- | ||
| + | | dd H<sub>2</sub>O || 5 | ||
| + | |} | ||
| + | |||
| + | :'''Conditions''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! colspan="3" | Biorad MyCycler* | ||
| + | |- | ||
| + | ! Cycles !! temperature [°C] !! Time [s] | ||
| + | |- | ||
| + | | 1 || 98 || 10 | ||
| + | |- | ||
| + | | rowspan="3"| 12 || 98 || 1 | ||
| + | |- | ||
| + | | 65 ↓ 0.5 || 5 | ||
| + | |- | ||
| + | | 72 || 1:00 | ||
| + | |- | ||
| + | | rowspan="3"| 18 || 98 || 1 | ||
| + | |- | ||
| + | | 63 || 5 | ||
| + | |- | ||
| + | | 72 || 1:00 | ||
| + | |- | ||
| + | | 1 || 72 || 5min | ||
| + | |- | ||
| + | | 1 || 12 || inf | ||
| + | |} | ||
| + | |||
| + | '''Results:''' | ||
| + | * Amplification of DelOP resulted in a small band atthe desired lenght, but also a smear and several unexpected bands | ||
| + | * bands were cut out and DNA purified using QIAquick Gel Extraction Kit | ||
| + | * amplicon will be used for restriction digest to validate the construct | ||
| + | |||
| + | ===Amplification from FS_22 to FS_13(s/l); 2.7 kb=== | ||
| + | |||
| + | :'''Reaction''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! what !! µl | ||
| + | |- | ||
| + | | ''D.acidovorans'' || 1 | ||
| + | |- | ||
| + | | FS_22: (1/10) || 2 | ||
| + | |- | ||
| + | | FS_13_short/FS_13_long: (1/10) || 2 | ||
| + | |- | ||
| + | | Phusion flash Master Mix || 10 | ||
| + | |- | ||
| + | |DMSO || 1/- | ||
| + | |- | ||
| + | | dd H<sub>2</sub>O || 4/5 | ||
| + | |} | ||
| + | |||
| + | :'''Conditions''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! colspan="3" | Biorad C1000 Touch | ||
| + | |- | ||
| + | ! Cycles !! temperature [°C] !! Time [s] | ||
| + | |- | ||
| + | | 1 || 98 || 10 | ||
| + | |- | ||
| + | | rowspan="2"| 30 || 98 || 1 | ||
| + | |- | ||
| + | | 72 || 1:00 | ||
| + | |- | ||
| + | | 1 || 72 || 5 min | ||
| + | |- | ||
| + | | 1 || 12 || inf | ||
| + | |} | ||
| + | |||
| + | :'''Conditions''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! colspan="3" | Biorad MyCycler* | ||
| + | |- | ||
| + | ! Cycles !! temperature [°C] !! Time [s] | ||
| + | |- | ||
| + | | 1 || 98 || 10 | ||
| + | |- | ||
| + | | rowspan="3"| 30 || 98 || 1 | ||
| + | |- | ||
| + | | 70 || 5 | ||
| + | |- | ||
| + | | 72 || 1:00 | ||
| + | |- | ||
| + | | 1 || 72 || 5 min | ||
| + | |- | ||
| + | | 1 || 12 || inf | ||
| + | |} | ||
| + | |||
==01-08-2013== | ==01-08-2013== | ||
Revision as of 20:23, 30 September 2013
Contents |
30-07-2013
Re-PCR of DelOP FS_22 to FS_13; 2.7 kb; 19-07-2013)
3x20µl
- Reaction
| what | µl |
|---|---|
| Fragment FS_22 to FS_13_short (19-07-2013) | 1 |
| FS_22: (1/10) | 2 |
| FS_13_long: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
- Amplification of DelOP did not work
- PCR will be repeated with different primer concentrations to estimate whether primer dimes might be the reason for insufficient amplification
Restriction digest of fragment FS_22 to FS_13; 2.7 kb; 25-07-2013) with EcoRI-HF
Incubation at 37°C for
| what | µl |
|---|---|
| FS_22 to FS_13long(25-07-2013) | 17 |
| EcoRI | 1 |
| Buffer CutSmart | 2 |
| Expected fragment lengths [bp] | 1883, 960 |
Amplification from FS_22 to FS_13s; 2.7 kb
2x20µl
- Reaction
| what | µl |
|---|---|
| D.acidovorans | 1 |
| FS_22: (1/10) | 4 |
| FS_13_short: (1/10) | 4 |
| Phusion flash Master Mix | 10 |
| dd H2O | 1 |
20µl
- Reaction
| what | µl |
|---|---|
| D.acidovorans | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
- Amplification of DelOP resulted in a small band atthe desired lenght, but also a smear and several unexpected bands
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- amplicon will be used for restriction digest to validate the construct
Amplification from FS_22 to FS_13(s/l); 2.7 kb
- Reaction
| what | µl |
|---|---|
| D.acidovorans | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short/FS_13_long: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1/- |
| dd H2O | 4/5 |
- Conditions
| Biorad C1000 Touch | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 1:00 | |
| 1 | 72 | 5 min |
| 1 | 12 | inf |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 70 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5 min |
| 1 | 12 | inf |
01-08-2013
Amplification from FS_22 to FS_13(s); 2.7 kb
- Reaction
| what | µl |
|---|---|
| D.acidovorans DSM-39 | 1 |
| FS_22: (1/10) | 2 |
| FS_13_short/FS_13_long: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 92 | 5 | |
| 91 | 5 | |
| 90 | 5 | |
| 89 | 5 | |
| 88 | 5 | |
| 87 | 5 | |
| 86 | 5 | |
| 85 | 5 | |
| 84 | 5 | |
| 82 | 5 | |
| 80 | 5 | |
| 78 | 5 | |
| 76 | 5 | |
| 74 | 5 | |
| 72 | 5 | |
| 70 | 5 | |
| 68 | 5 | |
| 66 | 5 | |
| 64 | 5 | |
| 62 | 5 | |
| 60 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5 min |
| 1 | 8 | inf |
Results:
- Amplification of DelOP did not work with stepwise cooling to the intended annealing temperature of 60°C
07-08-2013
Amplification from FS_22 to FS_13; 2.7 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_22: (1/10) | 2 |
| FS_13: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65 ↓ 0.5 | 5 | |
| 72 | 1:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Amplification from FS_22 to FS_13; 2.7 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_22: (1/10) | 2 |
| FS_13: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| dd H2O | 5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 55 | 5 | |
| 72 | 1:00 | |
| 1 | 72 | 5min |
| 1 | 12 | inf |
Results:
- Amplification of DelOP led to a band of the desired size as well as a smear and several different sideproducts
- Band was cut out and DNA purified using QIAquick Gel Extraction Kit to be restriction digested for validation of the PCR product
- Amplification will be repeated at lower temperature to obtain more product
 "
"