Team:Heidelberg/Tyrocidine week13 ms
From 2013.igem.org
m |
|||
| Line 1: | Line 1: | ||
| - | |||
==Amplifications== | ==Amplifications== | ||
===Amplification of fragment 1=== | ===Amplification of fragment 1=== | ||
====A==== | ====A==== | ||
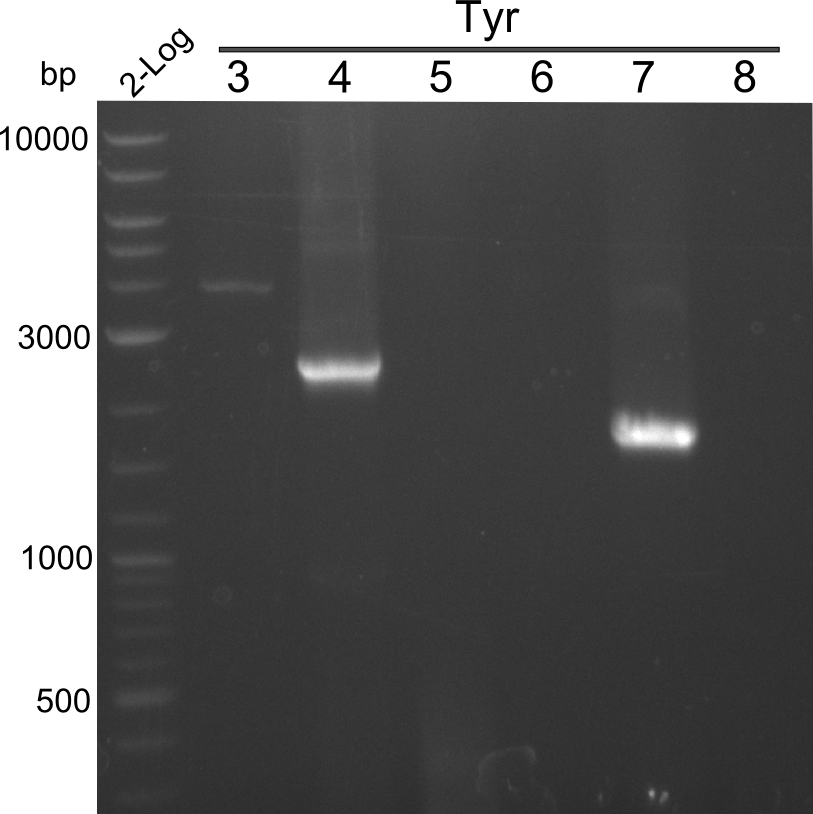
| - | [[File: | + | [[File:Heidelberg_20130725_4µL2log_tyr3-8.png|thumbnail|right|PCR Results of 25.07.13 |
2-log ladder and Tyr 3-8 with Q5]] | 2-log ladder and Tyr 3-8 with Q5]] | ||
| Line 43: | Line 42: | ||
====B==== | ====B==== | ||
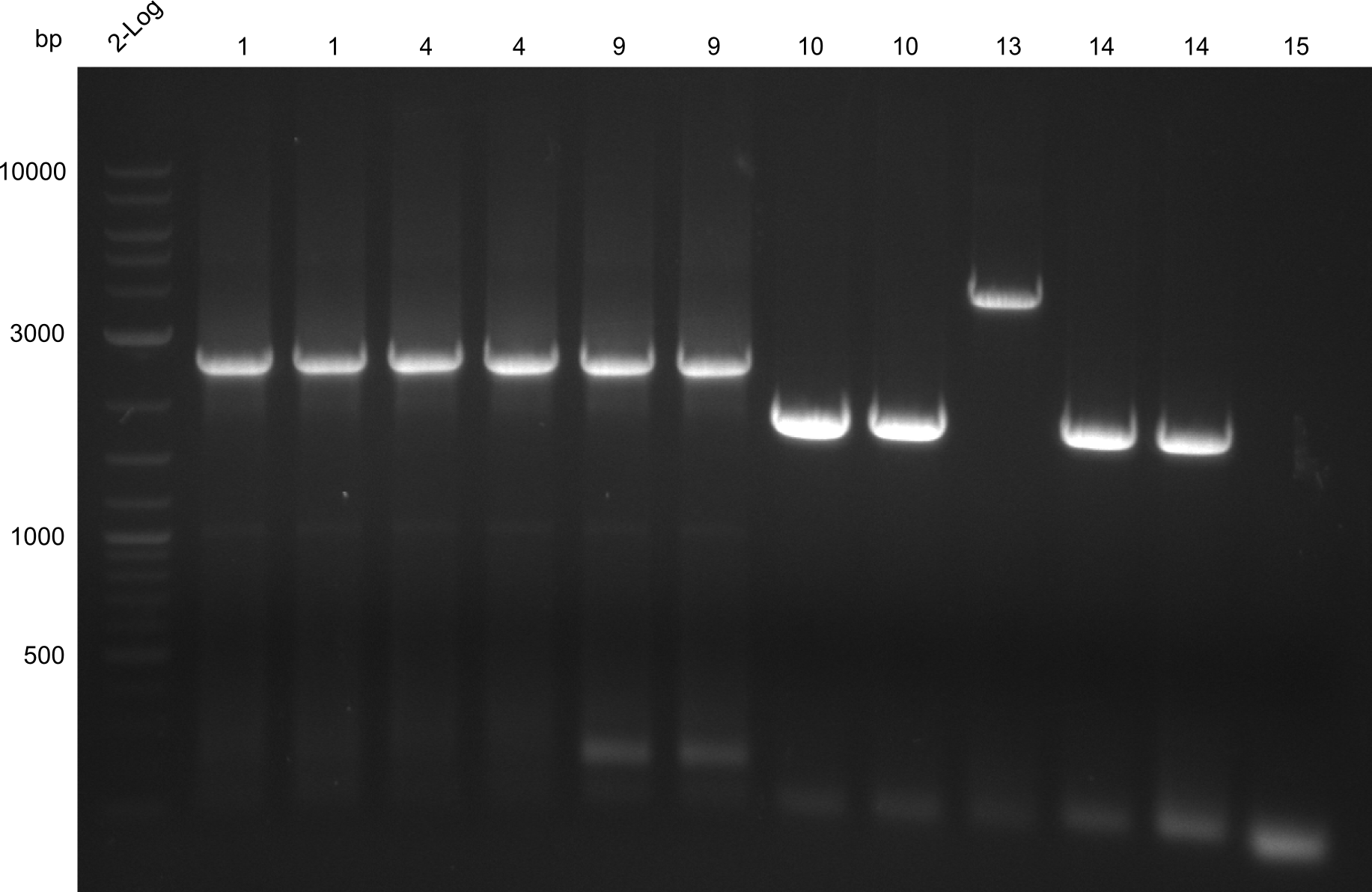
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 1,4,9,10,13,14,15.png|right|thumbnail|all constructs extracted by gel extraction; fragment 15 missing -> Optimization]] |
{| class="wikitable" | {| class="wikitable" | ||
| Line 80: | Line 79: | ||
===Amplification of fragment 2=== | ===Amplification of fragment 2=== | ||
====A==== | ====A==== | ||
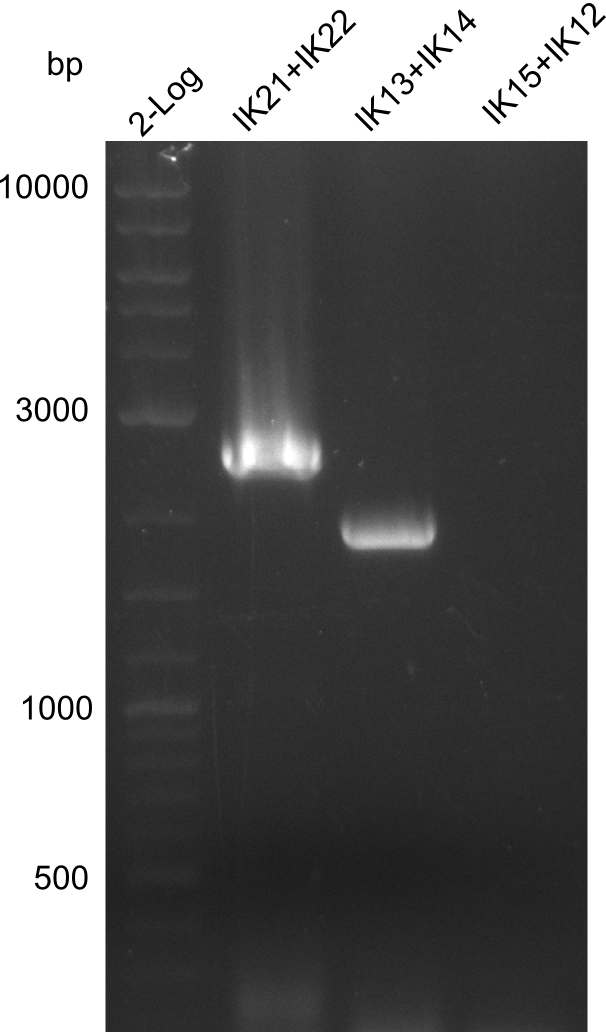
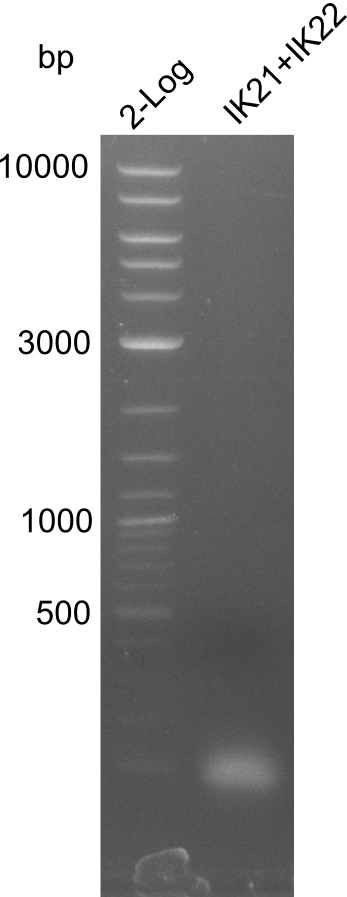
| - | [[File: | + | [[File:Heidelberg_B.brevis-PCR2013-07-24.png|right|thumb|100px| Lane 1: NEB 2-log; |
lane 2: pJM5 with primers IK21+IK22; lane 3: ''B. parabrevis'' with primers | lane 2: pJM5 with primers IK21+IK22; lane 3: ''B. parabrevis'' with primers | ||
| Line 122: | Line 121: | ||
====B==== | ====B==== | ||
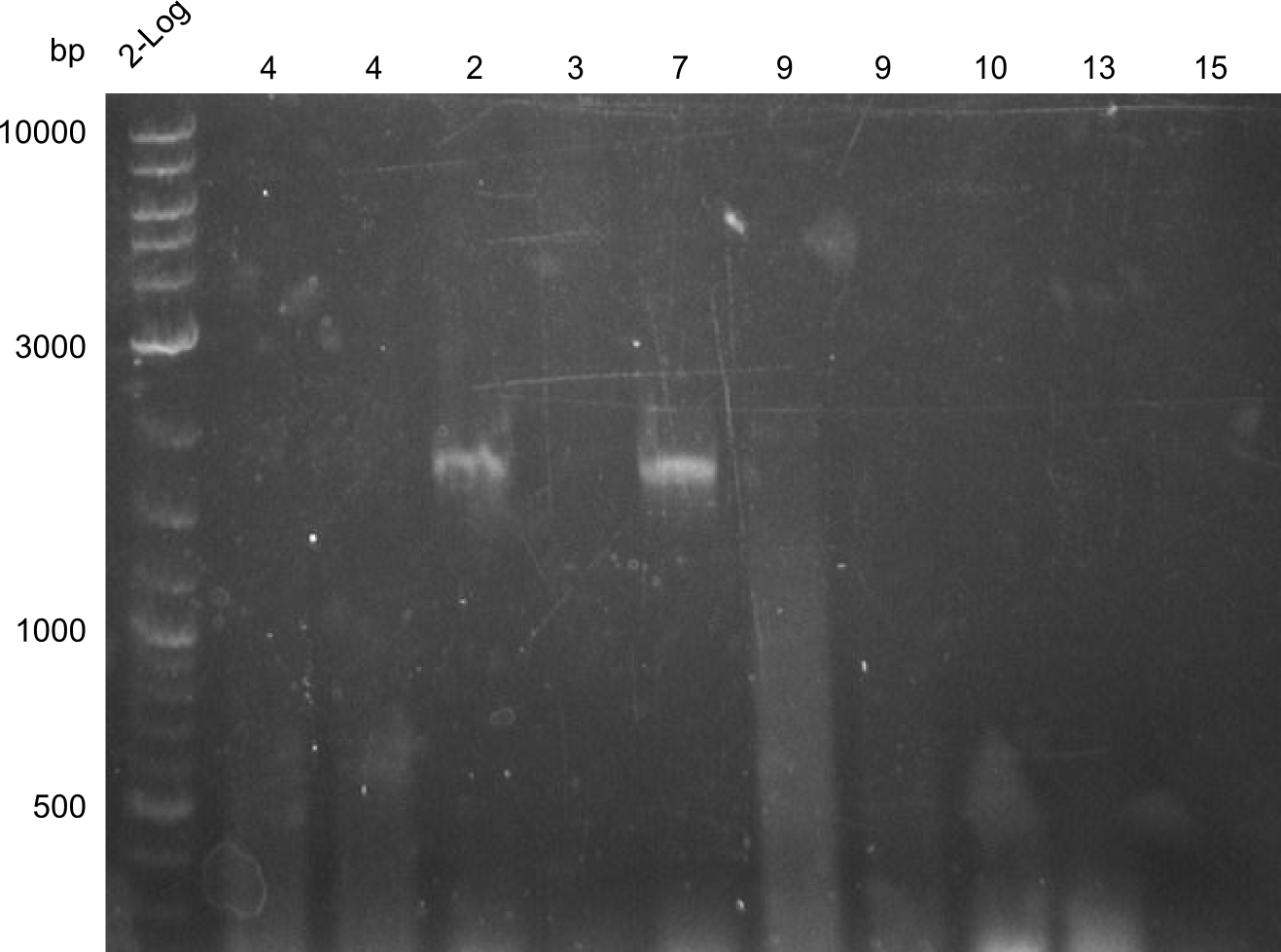
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 163: | Line 162: | ||
===Amplification of fragment 3=== | ===Amplification of fragment 3=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_B.brevis-PCR2013-07-24.png|right|thumb|100px| Lane 1: NEB 2-log; |
lane 2: pJM5 with primers IK21+IK22; lane 3: ''B. parabrevis'' with primers | lane 2: pJM5 with primers IK21+IK22; lane 3: ''B. parabrevis'' with primers | ||
| Line 204: | Line 203: | ||
no band; new PCR settings | no band; new PCR settings | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_20130725_4µl_log2_IK15toIK12.png|right|thumbnail|100px|Lane 1: NEB |
2-log; lane 2: ''B. parabrevis'' with fragment 3]] | 2-log; lane 2: ''B. parabrevis'' with fragment 3]] | ||
| Line 234: | Line 233: | ||
xxxxxxxx; new: touchdown PCR with Phusion Flash | xxxxxxxx; new: touchdown PCR with Phusion Flash | ||
====C==== | ====C==== | ||
| - | [[File: | + | [[File:Heidelberg_20130725_4µL2log_tyr3-8.png|thumbnail|right|PCR Results of 25.07.13 |
2-log ladder and Tyr 3-8 with Q5]] | 2-log ladder and Tyr 3-8 with Q5]] | ||
| Line 281: | Line 280: | ||
====D==== | ====D==== | ||
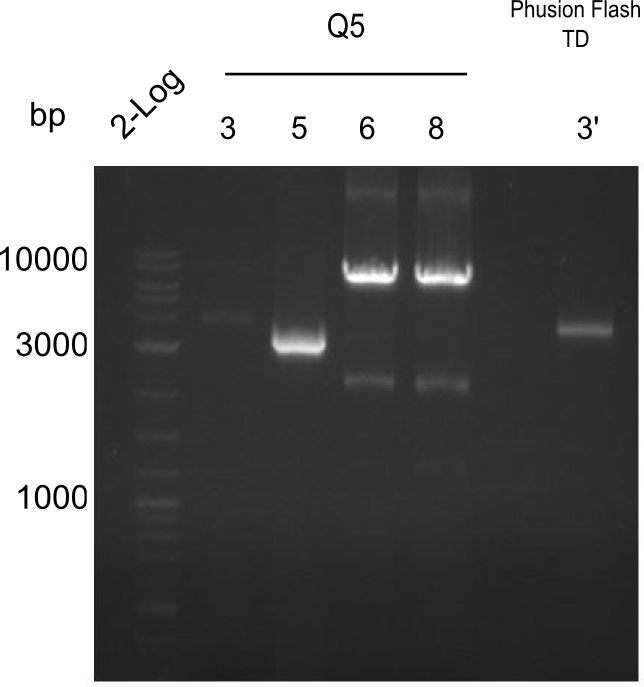
| - | [[File: | + | [[File:Heidelberg_20130726 2log TyrQ53 5 6 8 Ph 3 TC.png|thumbnail|right|PCR Results |
of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | ||
| Line 364: | Line 363: | ||
====F==== | ====F==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 404: | Line 403: | ||
===Amplification of fragment 4=== | ===Amplification of fragment 4=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_B.brevis-PCR2013-07-24.png|right|thumb|100px| Lane 1: NEB 2-log; |
lane 2: pJM5 with primers IK21+IK22; lane 3: ''B. parabrevis'' with primers | lane 2: pJM5 with primers IK21+IK22; lane 3: ''B. parabrevis'' with primers | ||
| Line 447: | Line 446: | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 488: | Line 487: | ||
====C==== | ====C==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 1,4,9,10,13,14,15.png|right|thumbnail|all constructs extracted by gel extraction; fragment 15 missing -> Optimization]] |
| Line 526: | Line 525: | ||
===Amplification of fragment 5=== | ===Amplification of fragment 5=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_20130725_4µL2log_tyr3-8.png|thumbnail|right|PCR Results of 25.07.13 |
2-log ladder and Tyr 3-8 with Q5]] | 2-log ladder and Tyr 3-8 with Q5]] | ||
| Line 561: | Line 560: | ||
|} | |} | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_20130726 2log TyrQ53 5 6 8 Ph 3 TC.png|thumbnail|right|PCR Results |
of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | ||
| Line 601: | Line 600: | ||
===Amplification of fragment 6=== | ===Amplification of fragment 6=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_20130725_4µL2log_tyr3-8.png|thumbnail|right|PCR Results of 25.07.13 |
2-log ladder and Tyr 3-8 with Q5]] | 2-log ladder and Tyr 3-8 with Q5]] | ||
| Line 637: | Line 636: | ||
|} | |} | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_20130726 2log TyrQ53 5 6 8 Ph 3 TC.png|thumbnail|right|PCR Results |
of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | ||
| Line 677: | Line 676: | ||
===Amplification of fragment 7=== | ===Amplification of fragment 7=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_20130725_4µL2log_tyr3-8.png|thumbnail|right|PCR Results of 25.07.13 |
2-log ladder and Tyr 3-8 with Q5]] | 2-log ladder and Tyr 3-8 with Q5]] | ||
| Line 713: | Line 712: | ||
|} | |} | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 754: | Line 753: | ||
===Amplification of fragment 8=== | ===Amplification of fragment 8=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_20130725_4µL2log_tyr3-8.png|thumbnail|right|PCR Results of 25.07.13 |
2-log ladder and Tyr 3-8 with Q5]] | 2-log ladder and Tyr 3-8 with Q5]] | ||
| Line 790: | Line 789: | ||
|} | |} | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_20130726 2log TyrQ53 5 6 8 Ph 3 TC.png|thumbnail|right|PCR Results |
of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and | ||
| Line 829: | Line 828: | ||
===Amplification of fragment 9=== | ===Amplification of fragment 9=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 868: | Line 867: | ||
'''PCR redone over night as gel was clumpy and did not show the expected fragments.''' | '''PCR redone over night as gel was clumpy and did not show the expected fragments.''' | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 1,4,9,10,13,14,15.png|right|thumbnail|all constructs extracted by gel extraction; fragment 15 missing -> Optimization]] |
{| class="wikitable" | {| class="wikitable" | ||
| Line 905: | Line 904: | ||
===Amplification of fragment 10=== | ===Amplification of fragment 10=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 979: | Line 978: | ||
===Amplification of fragment 11=== | ===Amplification of fragment 11=== | ||
====A==== | ====A==== | ||
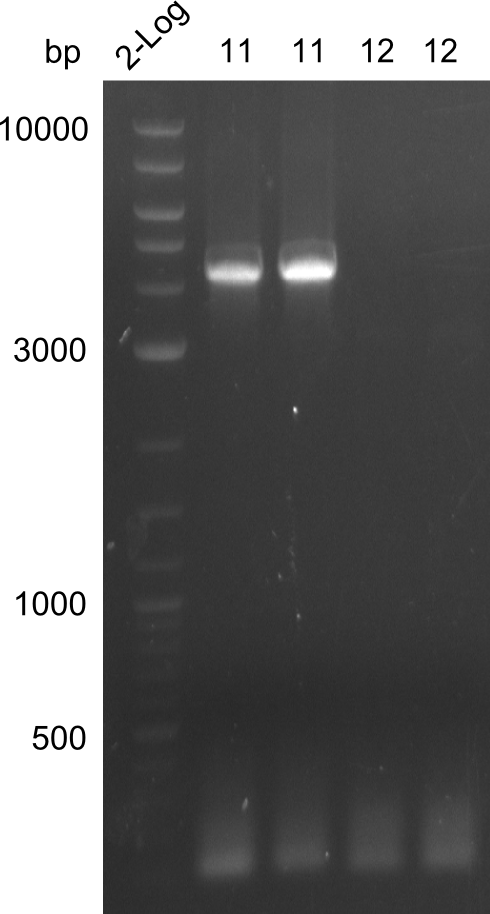
| - | [[File: | + | [[File:Heidelberg_20130729 2LOG Tyr11,12.png|right|thumbnail|amplification of fragments 11 & 12. Fragment 11 is clearly visible, fragment 12 is missing]] |
| Line 1,017: | Line 1,016: | ||
===Amplification of fragment 12=== | ===Amplification of fragment 12=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_20130729 2LOG Tyr11,12.png|right|thumbnail|amplification of fragments 11 & 12. Fragment 11 is clearly visible, fragment 12 is missing]] |
| Line 1,055: | Line 1,054: | ||
===Amplification of fragment 13=== | ===Amplification of fragment 13=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 1,093: | Line 1,092: | ||
'''PCR redone over night as gel was clumpy and did not show the expected fragments.''' | '''PCR redone over night as gel was clumpy and did not show the expected fragments.''' | ||
====B==== | ====B==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 1,4,9,10,13,14,15.png|right|thumbnail|all constructs extracted by gel extraction; fragment 15 missing -> Optimization]] |
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 1,129: | Line 1,128: | ||
===Amplification of fragment 14=== | ===Amplification of fragment 14=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 1,4,9,10,13,14,15.png|right|thumbnail|all constructs extracted by gel extraction; fragment 15 missing -> Optimization]] |
{| class="wikitable" | {| class="wikitable" | ||
| Line 1,168: | Line 1,167: | ||
===Amplification of fragment 15=== | ===Amplification of fragment 15=== | ||
====A==== | ====A==== | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine 2,3,4,7,9,10,13,15.png|thumbnail|right|PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction]] |
| Line 1,244: | Line 1,243: | ||
''2log ladder, 4 µL'' '''24.7:'''4, '''25.7:'''1,2,3,7, '''26.7.:'''3,5,6,8,3', '''28.7.'''1,2,4,9,10,13,14 ''2log ladder, 4 µL''<br> | ''2log ladder, 4 µL'' '''24.7:'''4, '''25.7:'''1,2,3,7, '''26.7.:'''3,5,6,8,3', '''28.7.'''1,2,4,9,10,13,14 ''2log ladder, 4 µL''<br> | ||
| - | [[File: | + | [[File:Heidelberg_Tyrocidine quantification gel.png|400px|right|thumbnail|quantification gel of the fragments extracted from gel. See table for precise amounts]] |
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 16:10, 4 October 2013
Amplifications
Amplification of fragment 1
A
| what | µl |
|---|---|
| pSB1C3 | 0,5 |
| IK22 | 2 |
| IK23 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5,5 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 120 |
| 35 | 98 | 5 |
| 66 | 10 | |
| 72 | 120 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result band in gel--> cut and extraction
B
| what | µl |
|---|---|
| IK23 | 2 |
| IK22 | 2 |
| pSB1C3 | 0.5 (for fragments 1, 4 & 9) |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 2
A
| what | µl |
|---|---|
| B. parabrevis | 1 |
| IK13 | 2 |
| IK14 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 300 |
| 35 | 98 | 5 |
| 70 | 10 | |
| 72 | 60 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result Successful
B
| what | µl |
|---|---|
| Bacillus | 1 |
| IK13 | 2 |
| IK14 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 70 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
Amplification of fragment 3
A
| what | µl |
|---|---|
| B. parabrevis | 1 |
| IK12 | 2 |
| IK15 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 300 |
| 35 | 98 | 5 |
| 66 | 10 | |
| 72 | 120 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result no band; new PCR settings
B
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 300 |
| 12 | 98 | 5 |
| 70 touchdown (-0.5°C) | 5 | |
| 72 | 180 | |
| 23 | 98 | 5 |
| 66 | 10 | |
| 72 | 180 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result xxxxxxxx; new: touchdown PCR with Phusion Flash
C
| what | µl |
|---|---|
| primer fw | 2 |
| primer rv | 2 |
| DMSO | 1 |
| B. parabrevis | 1 |
| Phusion flash | 10 |
| ddH20 | 4 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 120 |
| 12 | 98 | 5 |
| 70 touchdown (-0.5°C) | 5 | |
| 72 | 120 | |
| 23 | 98 | 5 |
| 64 | 5 | |
| 72 | 120 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result Just a light band, have to improve conditions.
D
Amplification with Q5
| what | µl |
|---|---|
| Bacillus | 1 |
| IK12 | 2 |
| IK15 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
E
Amplification with Phusion Flash
| what | µl |
|---|---|
| Bacillus | 1 |
| IK12 | 2 |
| IK15 | 2 |
| Phusion 2x Master mix | 10 |
| DMSO | 1 |
| ddH20 | 4 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 12 | 98 | 0:05 |
| 70↓0.5°C | 0:05 | |
| 72 | 2:30 | |
| 23 | 98 | 0:05 |
| 64 | 0:05 | |
| 72 | 3:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
F
| what | µl |
|---|---|
| Bacillus | 1 |
| IK12 | 2 |
| IK15 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 70 | 0:15 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
Amplification of fragment 4
A
| what | µl |
|---|---|
| pSB1C3 | 0,5 |
| IK21 (1:10) | 2 |
| IK22 (1:10) | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5,5 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 300 |
| 35 | 98 | 5 |
| 66 (fragment 4) | 10 | |
| 72 | 120 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result
Successful
B
| what | µl |
|---|---|
| pSB1C3 | 0.5 |
| IK21 | 2 |
| IK22 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Result PCR redone over night as gel was clumpy and did not show the expected fragments.
C
| what | µl |
|---|---|
| IK21 | 2 |
| IK22 | 2 |
| pSB1C3 | 0.5 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 5
A
| what | µl |
|---|---|
| B. Parabrevis | 1 |
| IK16 | 2 |
| IK17 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5,0 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 120 |
| 35 | 98 | 5 |
| 65 | 10 | |
| 72 | 60 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
B
| what | µl |
|---|---|
| Bacillus | 1 |
| IK16 | 2 |
| IK17 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 1:30 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 6
A
| what | µl |
|---|---|
| B. Parabrevis | 1 |
| IK12 | 2 |
| IK18 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5,0 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 120 |
| 35 | 98 | 5 |
| 66 | 10 | |
| 72 | 150 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
B
| what | µl |
|---|---|
| Bacillus | 1 |
| IK12 | 2 |
| IK18 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 3:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 7
A
| what | µl |
|---|---|
| B. Parabrevis | 1 |
| IK13 | 2 |
| IK19 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5,0 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 120 |
| 35 | 98 | 5 |
| 70 | 10 | |
| 72 | 60 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
B
| what | µl |
|---|---|
| Bacillus | 1 |
| IK13 | 2 |
| IK19 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 70 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
Amplification of fragment 8
A
| what | µl |
|---|---|
| B. Parabrevis | 1 |
| IK20 | 2 |
| IK12 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5,0 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 120 |
| 35 | 98 | 5 |
| 66 | 10 | |
| 72 | 150 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
B
| what | µl |
|---|---|
| Bacillus | 1 |
| IK12 | 2 |
| IK20 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 3:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 9
A
| what | µl |
|---|---|
| pSB1C3 | 0.5 |
| PW04 | 2 |
| IK21 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
B
| what | µl |
|---|---|
| PW04 | 2 |
| IK22 | 2 |
| pSB1C3 | 0.5 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 10
A
| what | µl |
|---|---|
| Bacillus | 1 |
| PW05 | 2 |
| PW06 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 70 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
B
| what | µl |
|---|---|
| PW05 | 2 |
| PW06 | 2 |
| Bacillus | 1 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 70 (fragment 10) | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 11
A
| what | µl |
|---|---|
| PW07 | 2 |
| PW08 | 2 |
| Bacillus | 1 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 72 | 0:15 | |
| 72 | 2:30 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 12
A
| what | µl |
|---|---|
| PW09 | 2 |
| PW10 | 2 |
| Bacillus | 1 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 69 | 0:15 | |
| 72 | 1:30 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 13
A
| what | µl |
|---|---|
| Bacillus | 1 (for fragments 2, 3, 7, 10, 13 & 15) |
| PW11 | 2 |
| IK12 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
B
| what | µl |
|---|---|
| PW11 | 2 |
| IK12 | 2 |
| Bacillus | 1 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 14
A
| what | µl |
|---|---|
| PW09 | 2 |
| IK12 | 2 |
| Bacillus | 1 (for fragments 2, 10, 13, 14 & 15) |
| pSB1C3 | 0.5 (for fragments 1, 4 & 9) |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 62 | 0:15 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Amplification of fragment 15
A
| what | µl |
|---|---|
| Bacillus | 1 (for fragments 2, 3, 7, 10, 13 & 15) |
| PW13 | 2 |
| IK12 | 2 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
PCR redone over night as gel was clumpy and did not show the expected fragments.
B
| what | µl |
|---|---|
| PW13 | 2 |
| IK12 | 2 |
| Bacillus | 1 |
| Q5 2x Master mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 2:00 |
| 35 | 98 | 0:05 |
| 66 | 0:15 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 12 | inf |
Analysis of DNA concentrations of previously amplified fragments
Analytical Gel Electrophoresis 0.8% agarose
Scheme: each 1µL DNA (eluation in 20µL water)+ 3 µL loading dye
2log ladder, 4 µL 24.7:4, 25.7:1,2,3,7, 26.7.:3,5,6,8,3', 28.7.1,2,4,9,10,13,14 2log ladder, 4 µL
| fragment | concentration [ng/µl] |
|---|---|
| 1 | 32 |
| 2 | 16 |
| 3 | -- |
| 4 | 30 |
| 5 | 12 |
| 6 | 8 |
| 7 | 12 |
| 8 | 8 |
| 9 | 30 |
| 10 | 40 |
| 11 | in progress |
| 12 | in progress |
| 13 | 4 |
| 14 | 40 |
| 15 | -- |
 "
"