From 2013.igem.org
(Difference between revisions)
|
|
| Line 1: |
Line 1: |
| - |
| |
| | == Tyrocidine-Indigoidine fusion - extended == | | == Tyrocidine-Indigoidine fusion - extended == |
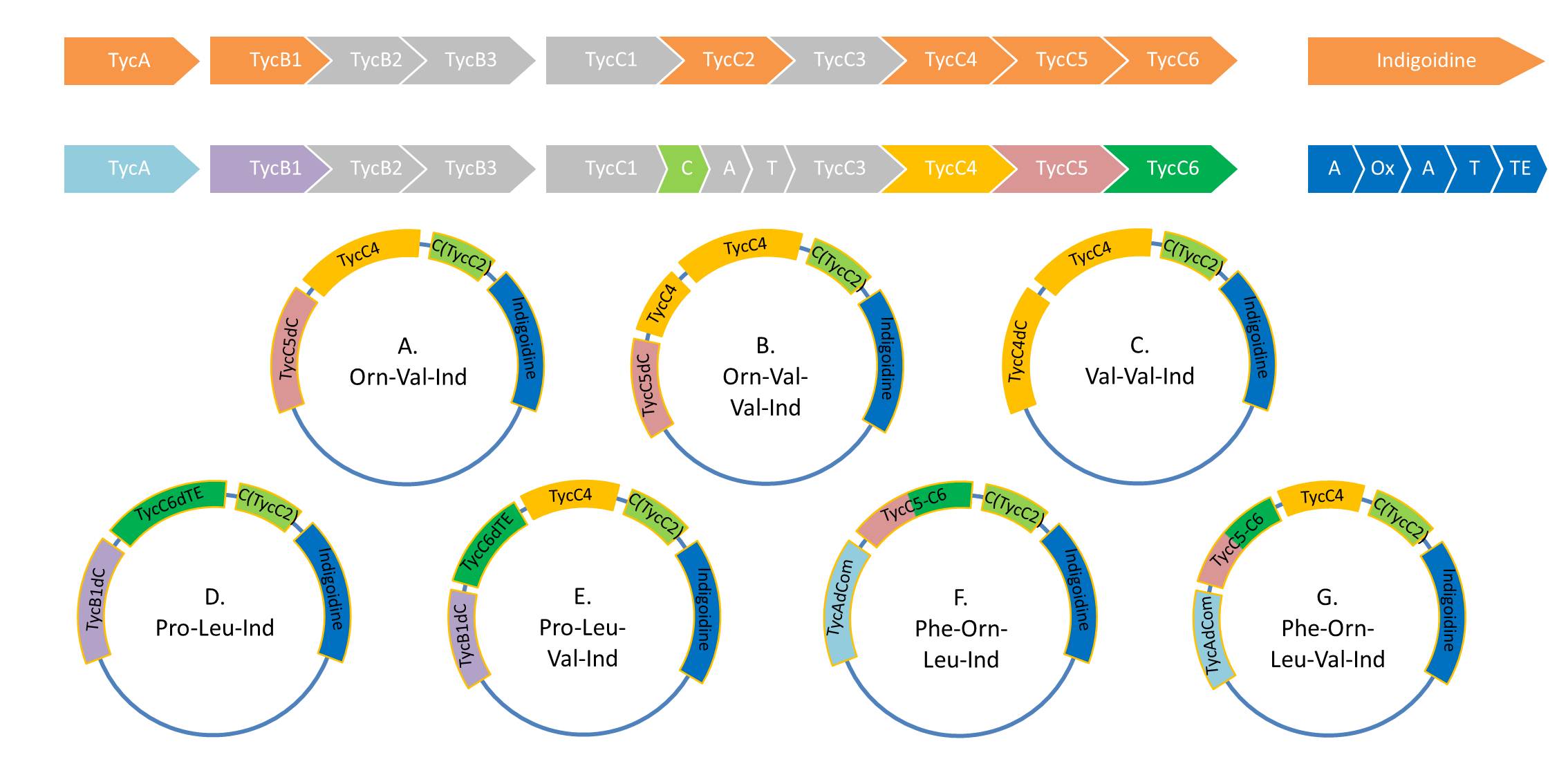
| | As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of Fusion-constructs in order to test Indigoidine as possible tag for NRPs and their synthesis. Herefore, we aim to build the following 7 NRPs: | | As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of Fusion-constructs in order to test Indigoidine as possible tag for NRPs and their synthesis. Herefore, we aim to build the following 7 NRPs: |
| | | | |
| - | [[File:Heidelberg_Tyc-Ind Fusion_extended.jpeg|1000px|center|thumb]] | + | [[File:Heidelberg_Tyc-Ind Fusion_extended.jpeg|300px|center|thumb]] |
| | | | |
| | === Amplifications === | | === Amplifications === |
Revision as of 17:32, 4 October 2013
Tyrocidine-Indigoidine fusion - extended
As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of Fusion-constructs in order to test Indigoidine as possible tag for NRPs and their synthesis. Herefore, we aim to build the following 7 NRPs:
Amplifications
Protocols
A
| what | µl
|
| pIK04 | 1
|
| PW26 | 2
|
| PW27 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 66 | 0:10
|
| 72 | 0:50
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
B
| what | µl
|
| B. parabrevis | 1
|
| PW28 | 2
|
| PW29 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 58 | 0:10
|
| 72 | 0:30
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
C
| what | µl
|
| pPW05 | 1
|
| PW30 | 2
|
| PW16 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 64 | 0:10
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
D
| what | µl
|
| pIK04 | 1
|
| PW37 | 2
|
| PW23 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 66 | 0:10
|
| 72 | 0:50
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
E
| what | µl
|
| B. parabrevis | 1
|
| PW28 | 2
|
| PW31 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 63 | 0:10
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
F
| what | µl
|
| B. parabrevis | 1
|
| PW32 | 2
|
| PW29 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 58 | 0:10
|
| 72 | 0:30
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
G
| what | µl
|
| pPW05 | 1
|
| PW19 | 2
|
| PW31 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 66 | 0:10
|
| 72 | 0:50
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
H
| what | µl
|
| pIK03 | 1
|
| IK13 | 2
|
| PW33 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 65 | 0:10
|
| 72 | 1:40
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
I
| what | µl
|
| pPW05 | 1
|
| PW34 | 2
|
| PW16 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 60 | 0:10
|
| 72 | 1:40
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
J
| what | µl
|
| pIK04 | 1
|
| IK23 | 2
|
| PW23 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 66 | 0:10
|
| 72 | 0:50
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
K
| what | µl
|
| pIK03 | 1
|
| IK13 | 2
|
| PW35 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 65 | 0:10
|
| 72 | 1:40
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
L
| what | µl
|
| B. parabrevis | 1
|
| PW36 | 2
|
| PW29 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 58 | 0:10
|
| 72 | 0:30
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
M
| what | µl
|
| pIK04 | 1
|
| IK16 | 2
|
| PW33 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 65 | 0:10
|
| 72 | 3:10
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
N
| what | µl
|
| pIK04 | 1
|
| IK16 | 2
|
| PW35 | 2
|
| Phusion Flash 2x Master Mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 35 | 98 | 0:05
|
| 65 | 0:10
|
| 72 | 3:10
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
Gel-Pictures
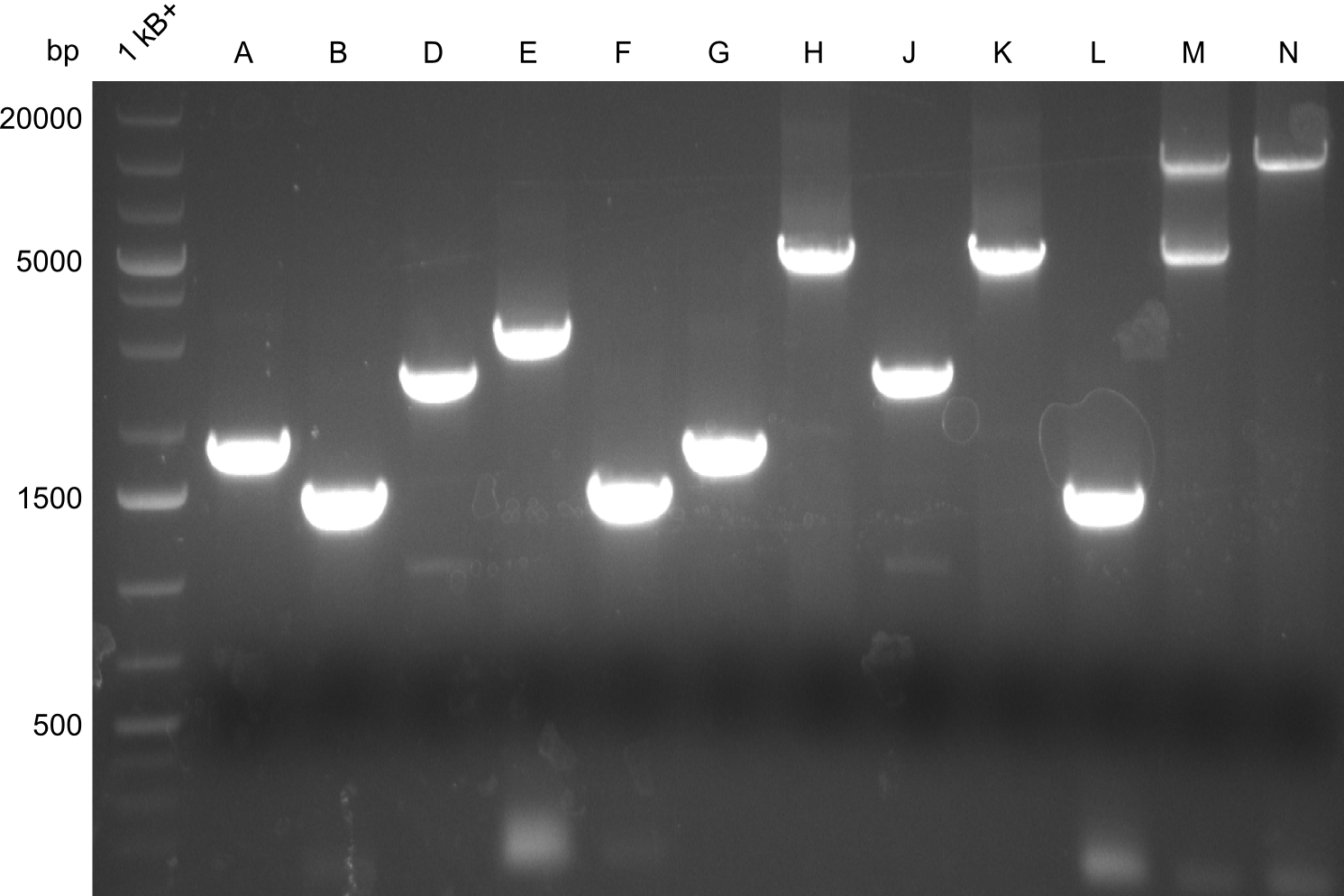
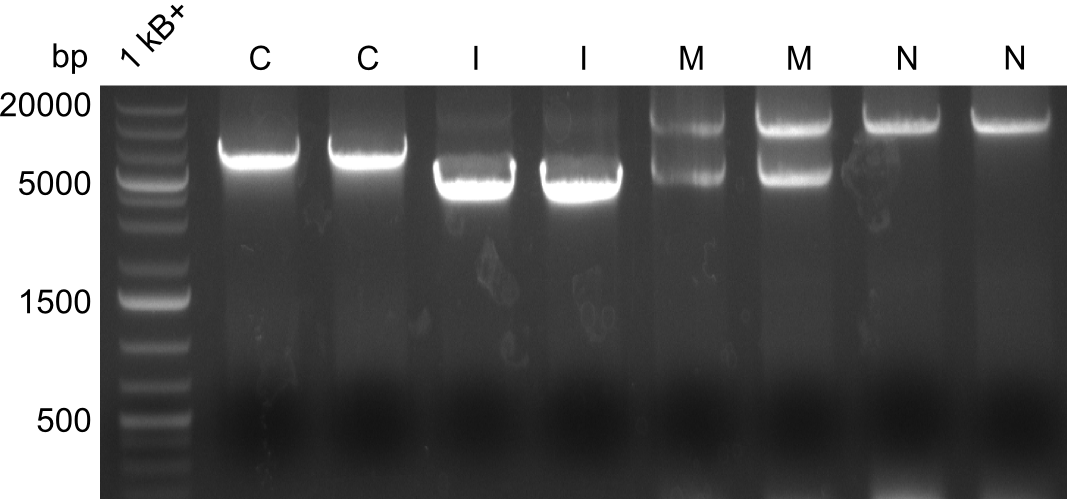
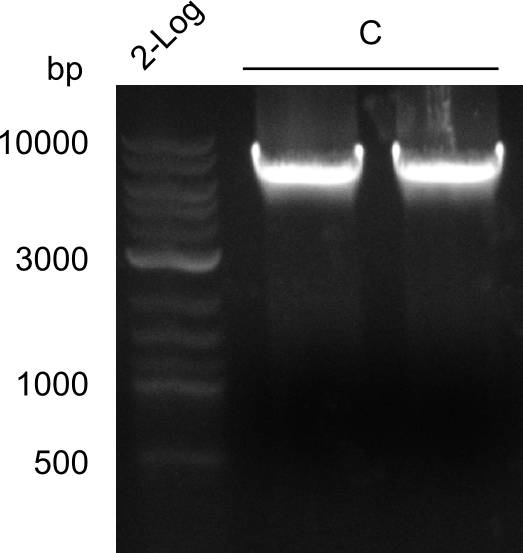
Quantification-Gel
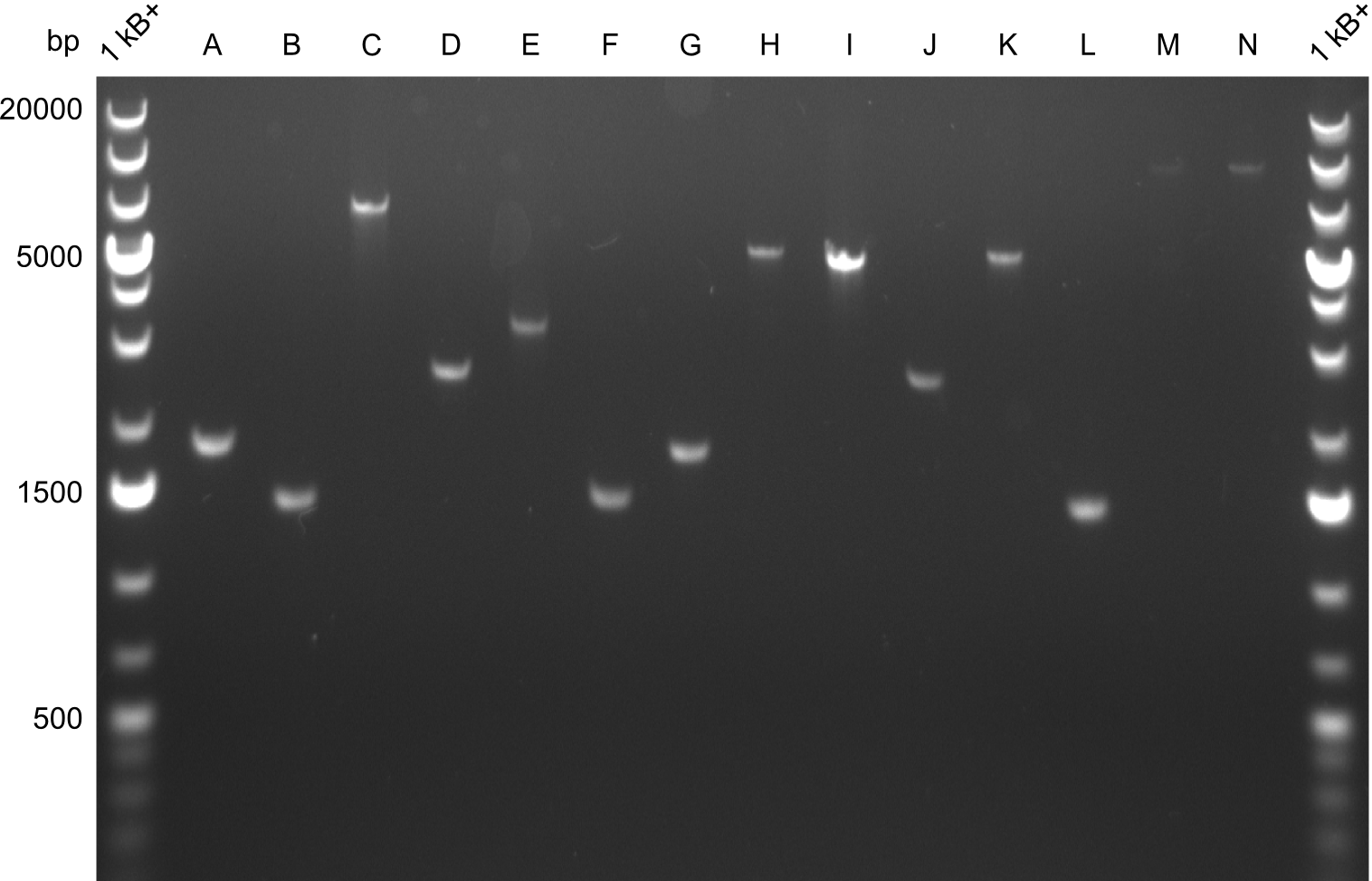
Gibson Assembly
pPW06 (Orn-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| A | 40 | 1.61
|
| B | 35 | 0.92
|
| C | 30 | 6.97
|
| D | 40 | 0.50
|
pPW07 (Orn-Val-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| A | 40 | 1.16
|
| C | 30 | 4.32
|
| D | 40 | 0.36
|
| E | 20 | 3.49
|
| F | 35 | 0.66
|
pPW08 (Val-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| C | 30 | 6.07
|
| F | 35 | 0.93
|
| G | 40 | 1.63
|
| OldG | 15 | 1.36
|
pPW09 (Pro-Leu-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| H | 20 | 6.35
|
| I | 40 | 3.02
|
| J | 25 | 0.63
|
pPW10 (Pro-Leu-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| C | 30 | 4.58
|
| J | 20 | 0.66
|
| K | 25 | 4.23
|
| L | 40 | 0.53
|
pPW11 (Phe-Orn-Leu-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| I | 40 | 0.58
|
| J | 20 | 0.15
|
| M | 5 | 9.27
|
pPW12 (Phe-Orn-Leu-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL]
|
| C | 30 | 1.77
|
| J | 20 | 0.26
|
| L | 40 | 0.20
|
| N | 10 | 7.77
|
Cells were transformed with 1 µl and 14 µl 1:2 diluted Gibson-Mix.
Screening for positive transformants
Colony-PCR
Screening for the Tyrocidine-part with VF2 and PW14
|
Screening for the Indigoidine-part with VR and KH05
|
First, there were colonies visible for the transformants of pPW09, pPW11 and pPW12. Later colonies for pPW06 and pPW10 followed.
Screening for the Tyrocidine-part with VF2 and PW14]]
|
Screening for the Indigoidine-part with VR and KH05]]
|
Restriction Digest
Positive samples were digested with restriction digest with EcoRI. The expected sizes of bands were:
- pPW06:
- pPW09:
- pPW10:
- pPW11:
digest with EcoRI after 20 min on gel]]
|
digest with EcoRI after 40 min on gel]]
|
After only 20 minutes of running, the bands were not yet clearly separated. Waiting for another 20 minutes lead to a interpretable result.
 "
"