Team:Buenos Aires/ res basu
From 2013.igem.org
| Line 40: | Line 40: | ||
| - | + | {| class="wikitable" style="background-color:#fff; margin:auto;" | |
| - | + | |+Results | |
| - | + | |- | |
| - | [[File:Carlotto1.JPG|center|600px]] | + | |[[File:Carlotto1.JPG|center|600px]] |
Fluorescence microscope picture 0 minutes after sealing | Fluorescence microscope picture 0 minutes after sealing | ||
| - | + | |- | |
| - | + | | | |
| - | + | ||
| - | + | ||
| - | + | ||
[[File:Carlotto15min.JPG|center|600px]] | [[File:Carlotto15min.JPG|center|600px]] | ||
Fluorescence microscope picture 15 minutes after sealing | Fluorescence microscope picture 15 minutes after sealing | ||
| - | + | |- | |
| - | + | |[[File:Carlotto30min.JPG|center|600px]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[File:Carlotto30min.JPG|center|600px]] | + | |
Fluorescence microscope picture 30 minutes after sealing | Fluorescence microscope picture 30 minutes after sealing | ||
| - | + | |- | |
| - | + | |[[File:Carlotto45min.JPG|center|600px]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[File:Carlotto45min.JPG|center|600px]] | + | |
Fluorescence microscope picture 45 minutes after sealing | Fluorescence microscope picture 45 minutes after sealing | ||
| - | + | |- | |
| - | + | | | |
| - | + | ||
| - | + | ||
| - | + | ||
[[File:Carlotto60min.JPG|center|600px]] | [[File:Carlotto60min.JPG|center|600px]] | ||
Fluorescence microscope picture 60 minutes after sealing | Fluorescence microscope picture 60 minutes after sealing | ||
| + | |||
| + | |} | ||
| + | |||
Revision as of 19:04, 26 October 2013
Contents |
Response of the construction http://parts.igem.org/Part:BBa_K1166000 Bba_K1166000 under different hypoxia conditions
Objective
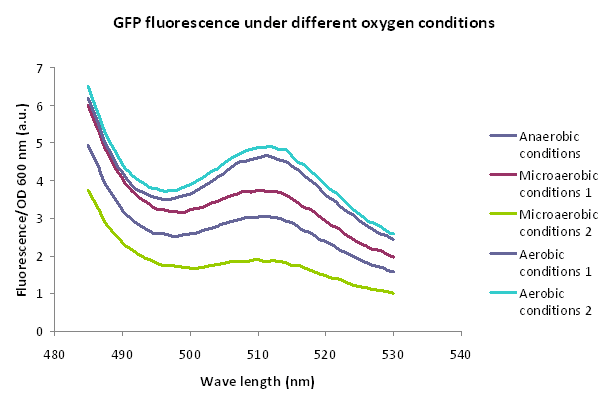
We aimed to characterize the part sent by de Monterrey Team which is a GFP under a hypoxia inducible promoter, cloned in a replicative plasmid (Bba_K1166000). For that purpose we transformed E. coli DH5α with plamid Bba_K1166000 and conducted 2 different assays described below using this strain.
Experiment 1: hypoxia induced by anaerobiosis boxes
Method
A 5ml culture of E. coli DH5α carrying the plasmid Bba_K1166000 in LB medium was grown for 16 h in aerobiosis, 1 ml was centrifuged and the pellet was kept frozen in order to use it as fluorescence control without induction. The rest of the culture was incubated for 20 h under anaerobic and microanaerobic conditions using hermetic boxes and GENbox aner and GENbox microanaer systems (bioMérieux), respectively, without shacking at 37°C. GENbox aner and GENbox microanaer systems are based on a chemical compound that traps oxygen. One ml of each condition was centrifuged and the pellets frozen. Finally, fluorescence (excitation: 475 nm) was measure and normalized by OD600nm. Both treatments were performed in duplicate.
Results
Experiment 2: hipoxia induced in a microscope slide sealed
Method
An E. coli DH5α carrying the plasmid Bba_K1166000 was grown under aerobic conditions overnight. Then, 10ul of this culture was placed in a microscope slide, covered with a coverslip and sealed with nail enamel (similar as usually performed to induce hypoxia in plant cells).
Afterwards, pictures of bacteria were taken in a fluorescence microscope every 15 min for 1 h. The first picture was taken immediately after sealing. Every picture was taken using the same exposition time (1/5 sec). Unfortunately, a lot of bleaching was observed. To avoid this, the field was changed before taking each photo. From 0 min, bacteria exhibited green fluorescence, however no significant induction can be observed in the time points analyzed (15, 30, 45 and 60 min).
|
Fluorescence microscope picture 0 minutes after sealing |
|
Fluorescence microscope picture 15 minutes after sealing |
|
Fluorescence microscope picture 30 minutes after sealing |
|
Fluorescence microscope picture 45 minutes after sealing |
|
Fluorescence microscope picture 60 minutes after sealing |
Overall conclusions
Based on the results obtained, GFP is beeing produced. However, FNR-HIP1 promoter doesn't seem to respond to the oxygen concentration in growth medium. The fluorescence microscopy assay shows no variation between different oxygen conditions. Moreover, in the other experiment, GFP seems to be produced in higher amounts when bacteria were grown in the presence of oxygen.
 "
"