Team:DTU-Denmark/Notebook/19 August 2013
From 2013.igem.org
(→Conclusion) |
|||
| Line 86: | Line 86: | ||
|} | |} | ||
| - | === | + | ===PCR on one of the Hello Wolrd constructs to confirm it's intact=== |
| - | Performed | + | Performed PCR on the construct containing Sec2 to check the template is correct because our PCRs on it are not working. |
template: Sec2 construct | template: Sec2 construct | ||
| Line 179: | Line 179: | ||
* pZA21::RFP::araBAD 2 uL MgCl2 | * pZA21::RFP::araBAD 2 uL MgCl2 | ||
* negative pZA21::RFP::araBAD | * negative pZA21::RFP::araBAD | ||
| - | * | + | * check Sec2 |
| - | * | + | * check Sec2 (duplicate) |
| - | * negative | + | * negative check Sec2 |
* screening A1 | * screening A1 | ||
* screening A2 | * screening A2 | ||
Revision as of 15:51, 20 August 2013
19 August 2013
Navigate to the Previous or the Next Entry
Contents |
lab 208
Main purpose
Who was in the lab
Kristian, Henrike, Julia
Procedure
Preparation of constructs to send for sequencing
Sec2, TAT3-2, TAT3-1a, CycAX and HAO constructs were prepared for sequencing. Sample preparation was performed following the indications given by the EZ-Seq service by Macrogen:
5uL of 100~150ng/ul plasmid + 5uL of 5uM primers.
An overview of the sample composition can be found in the following link: File:Construct for sequencing - Sheet1.pdf
The samples were sent to Macrogen for sequencing.
Gradient PCR for creation of Biobricks
Made a screening to find good conditions for the extraction of our constructs for subsequent ligation into BioBricks.
conditions:
A:
- HF buffer
- 2% DMSO
- 1uL 50mM MgCl2
B:
- HF buffer
- 3% DMSO
- 2uL 50mM MgCl2
C:
- HF buffer
- 5% DMSO
- 1uL 50mM MgCl2
D:
- HF buffer
- 3% DMSO
- 1uL 50mM MgCl2
The annealing temperature was a gradient from 48C to 63C in 12 steps.
| sample # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| temperature | 48.3 | 48.7 | 50.0 | 51.8 | 53.3 | 54.7 | 56.3 | 57.5 | 59.1 | 60.8 | 62.5 | 63 |
PCR to linearize pZA21::RFP::araBAD
Used GC buffer and 5% DMSO. Made three samples with varying concentrations of 50mM MgCl2: 0,5 uL, 1uL and 2 uL.
template: pZA21::RFP::araBAD
primers: 55a, 55b
program:
| temperature | time | cycles |
|---|---|---|
| 98C | 2:00 | - |
| 98C | 0:10 | 36 |
| 65C | 1:00 | 36 |
| 72C | 3:00 | 36 |
| 72C | 5:00 | - |
| 10C | hold | - |
PCR on one of the Hello Wolrd constructs to confirm it's intact
Performed PCR on the construct containing Sec2 to check the template is correct because our PCRs on it are not working.
template: Sec2 construct
primers: sequencing primers 4_FW_pZA21 and 5_RV_pZA21
| temperature | time | cycles |
|---|---|---|
| 98C | 10:00 | - |
| 98C | 0:10 | 36 |
| 61C | 0:20 | 36 |
| 72C | 2:00 | 36 |
| 72C | 5:00 | - |
| 10C | hold | - |
PCR on NIR1 and NIR2 via. colony PCR for template to Morten Nørholm
| sample N1 or N2# | HF | HF 2% DMSO 1mL MgCl2 50mM | HF 5% DMSO | HF 1M Betaine | GC | GC 2% DMSO | GC 5% DMSO | GC 5% DMSO 1mL MgCl2 50mM | GC 5% DMSO 2mL MgCl2 50mM | GC 1M Betaine |
| temperature | time | cycles |
|---|---|---|
| 98C | 10:00 | - |
| 98C | 0:20 | 14 |
| 71C -0.5C increment | 0:30 | 14 |
| 72C | 2:30 | 14 |
| 98C | 0:20 | 20 |
| 64C | 0:30 | 20 |
| 72C | 2:30 | 20 |
| 72C | 5:00 | - |
| 10C | hold | - |
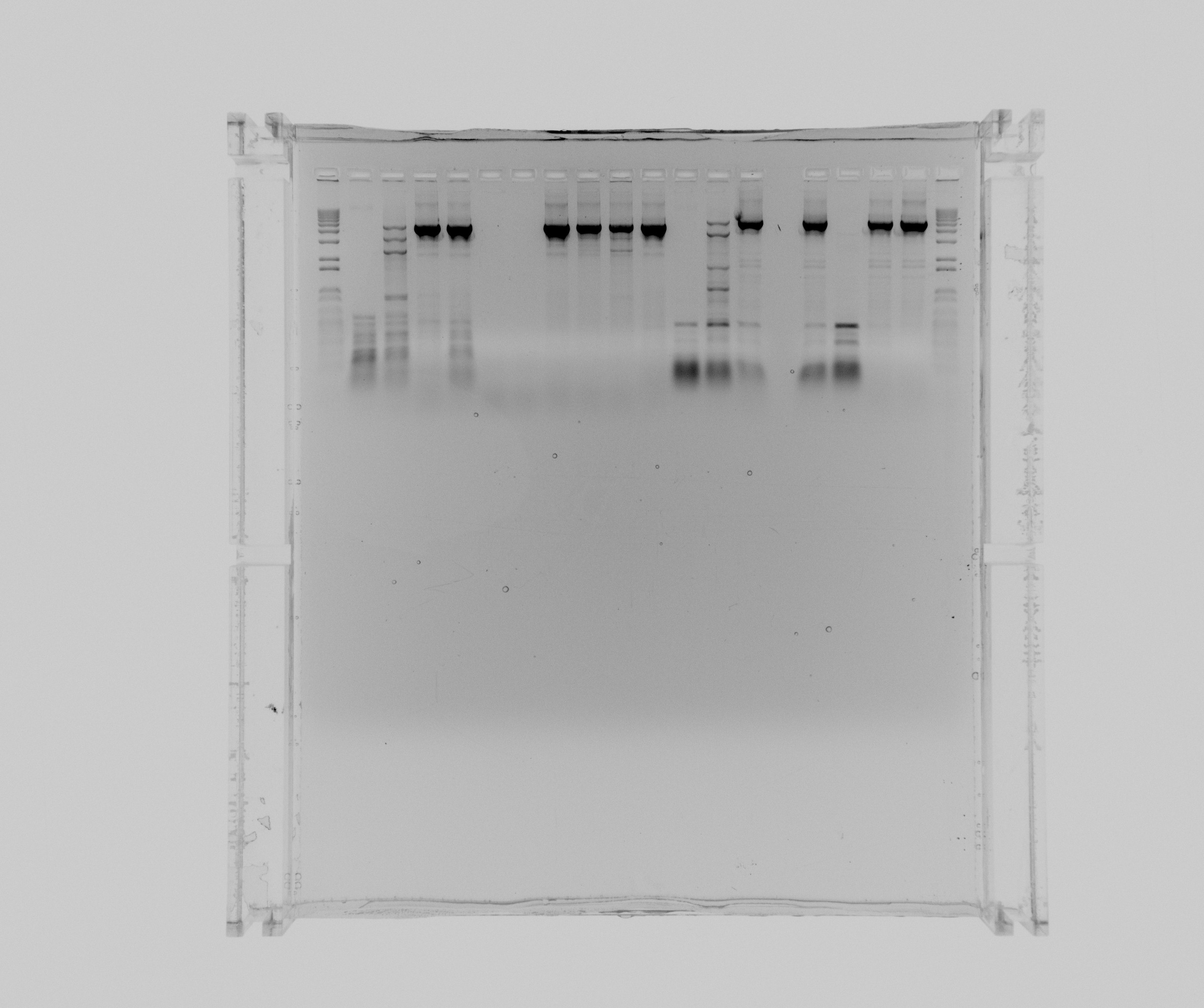
Results
gels
- 1: 1 kb ladder
- 2: N1 HF
- 3: N1 HF 2% DMSO 1 mL MgCl2
- 4: N1 HF 5% DMSO
- 5: N1 HF 1M Betaine
- 6: N1 GC
- 7: N1 GC 2% DMSO
- 8: N1 GC 5% DMSO
- 9: N1 GC 5% DMSO 1 mL MgCL2
- 10: N1 GC 5% DMSO 2 mL MgCl2
- 11: N1 GC 1M Betaine
- 12: N2 HF
- 13: N2 HF 2% DMSO 1 mL MgCl2
- 14: N2 HF 5% DMSO
- 15: N2 HF 1M Betaine
- 16: N2 GC
- 17: N2 GC 2% DMSO
- 18: N2 GC 5% DMSO
- 19: 1 kb ladder
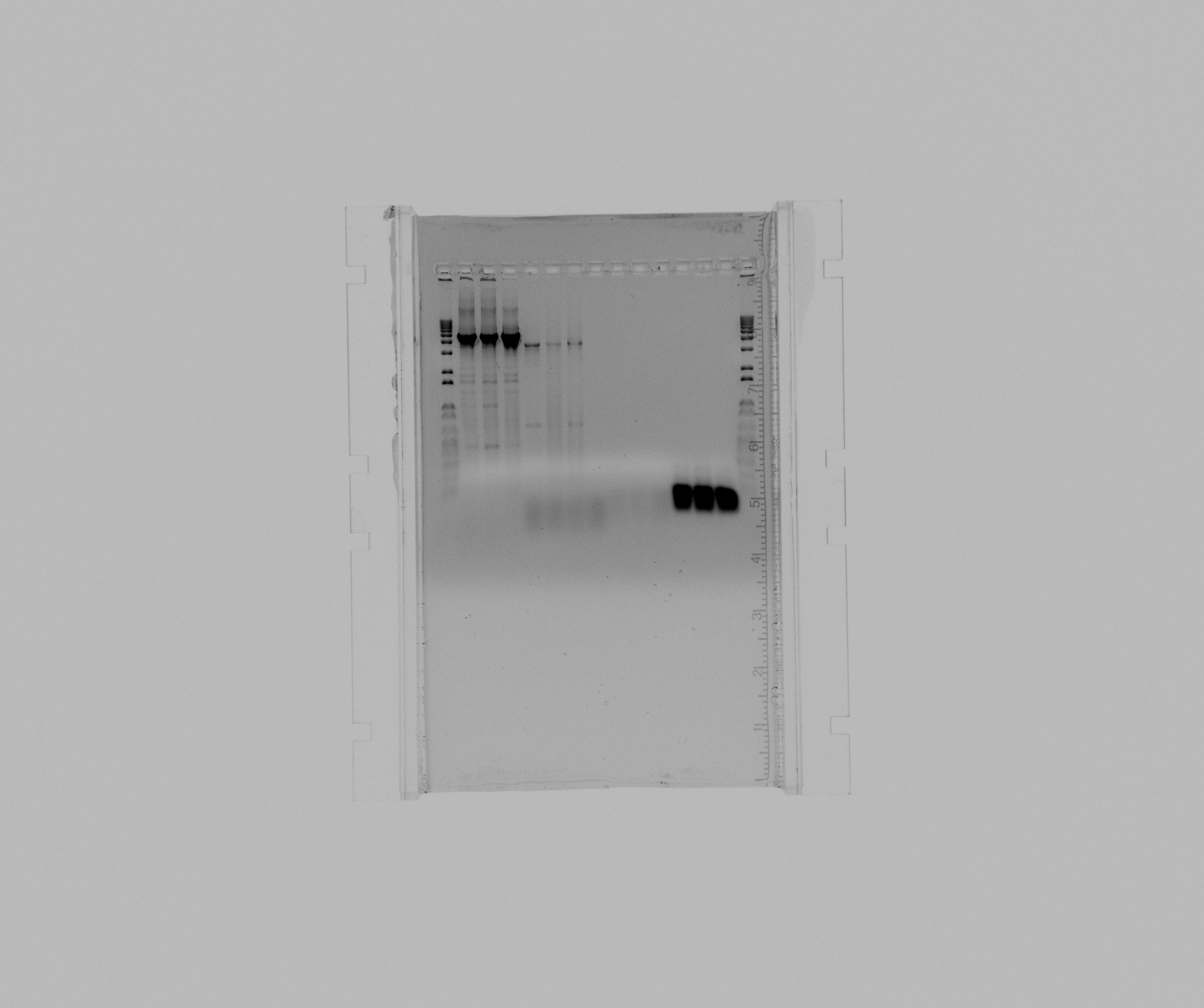
- 1 kb ladder
- N2 GC 5% DMSO 1 mL MgCl2
- N2 GC 5% DMSO 2 mL MgCl2
- N2 GC 1M Betaine
- pZA21::RFP::araBAD 0,5 uL MgCl2
- pZA21::RFP::araBAD 1 uL MgCl2
- pZA21::RFP::araBAD 2 uL MgCl2
- negative pZA21::RFP::araBAD
- check Sec2
- check Sec2 (duplicate)
- negative check Sec2
- screening A1
- screening A2
- screening A3
Conclusion
Navigate to the Previous or the Next Entry The program for Nir colony PCR works as a touchdown PCR and it have a big influence if you add DMSO or Betaine. It seems as 5% DMSO and no additional MgCl2 or 1M Betaine works best and somewhat equally good for both reactions.
 "
"