Team:Yale/Project Bioassay
From 2013.igem.org
(Difference between revisions)
(→Aims for the Project) |
(→Aims for the Project) |
||
| Line 23: | Line 23: | ||
=== Develop bioassay to screen PLA production === | === Develop bioassay to screen PLA production === | ||
*We needed a way to detect the PLA once we produced it using the heterologous enzymes | *We needed a way to detect the PLA once we produced it using the heterologous enzymes | ||
| - | **We decided to use the fluorescent dye Nile red, a intercellular lipid strain <br> | + | **We decided to use the fluorescent dye Nile red, a intercellular lipid strain |
| + | **Nile red does not affect the growth of bacteria, and its fluorescence is quenched in water <br> | ||
<center>[[File:Nile_red_PHB_stain.jpg|200px]]</center><br><br> | <center>[[File:Nile_red_PHB_stain.jpg|200px]]</center><br><br> | ||
| Line 29: | Line 30: | ||
*We used the 2012 Tokyo Tech Biobrick (BBa_K934001) as a positive control to attempt to detect Nile red fluorescence on our plate reader (ex. 530nm, em. 590nm) | *We used the 2012 Tokyo Tech Biobrick (BBa_K934001) as a positive control to attempt to detect Nile red fluorescence on our plate reader (ex. 530nm, em. 590nm) | ||
**This plasmid had the enzyme to synthesize P(3HB) a similar plastic PLA | **This plasmid had the enzyme to synthesize P(3HB) a similar plastic PLA | ||
| + | **Cells were grown for 24 hours in the presence of Nile red. The cells were washed and resuspended in PBS. | ||
<center>[[File:Tokyo Tech plasmid.jpg|400px]]</center><br><br> | <center>[[File:Tokyo Tech plasmid.jpg|400px]]</center><br><br> | ||
====Our Construct==== | ====Our Construct==== | ||
*We then proceeded to test out plasmid in the plate reader. | *We then proceeded to test out plasmid in the plate reader. | ||
| - | **Cells were grown for 24 with both enzymes induced and in the presence of Nile red. The cells were washed and resuspended in PBS. The readings were normalized for optical density. | + | **Cells were grown for 24 hours with both enzymes induced and in the presence of Nile red. The cells were washed and resuspended in PBS. The readings were normalized for optical density. |
| - | [[File:EcNR2 LB x20 wiki.jpg]] | + | <center>[[File:EcNR2 LB x20 wiki.jpg|400px]]</center><br><br> |
| - | <center>[[File:FACS.jpg|400px]]</center> | + | === FACS Sorting === |
| + | *In order to quickly screen the large diversity we planed to create using MAGE, we wanted to employ Fluorescence-activated cell sorting (FACS) | ||
| + | **FACS could sort cells based on the Nile red fluorescence, thus indicating those cells that have produced larger quantities of PLA | ||
| + | <center>[[File:FACS.jpg|400px]]</center><br><br> | ||
| + | |||
| + | *Nile red has an emission maximum at 598nm when bound to p(3HB) granules according to Spiekermann et al. 1998 | ||
| + | *Thus we decided to use PE-Texas Red which has an emission maximum at 615nm in order pick the best colonies. | ||
Revision as of 04:10, 31 August 2013
| Project Overview | Validate PLA synthesis | Develop bioassay | Apply MAGE | Introduce export system | Make a bioplastic |
|---|
Contents |
Aims for the Project
- Engineer strains of E. coli to validate PLA synthesis
- Develop bioassay to screen PLA production
- Apply MAGE to optimize PLA production, guided by FBA
- Introduce type 1 secretion system to export and extract PLA
- Make a bioplastic
Develop bioassay to screen PLA production
- We needed a way to detect the PLA once we produced it using the heterologous enzymes
- We decided to use the fluorescent dye Nile red, a intercellular lipid strain
- Nile red does not affect the growth of bacteria, and its fluorescence is quenched in water

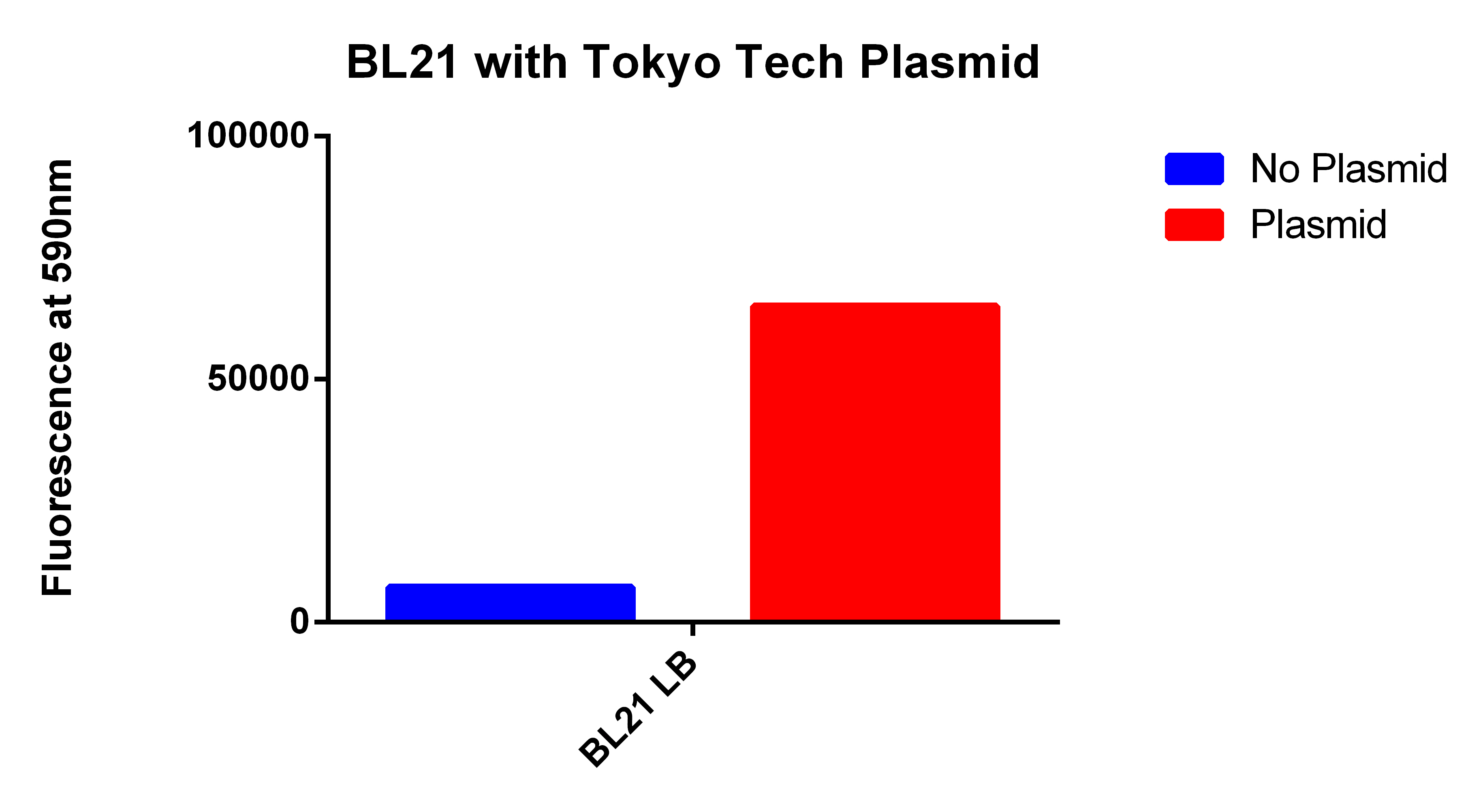
Positive Control
- We used the 2012 Tokyo Tech Biobrick (BBa_K934001) as a positive control to attempt to detect Nile red fluorescence on our plate reader (ex. 530nm, em. 590nm)
- This plasmid had the enzyme to synthesize P(3HB) a similar plastic PLA
- Cells were grown for 24 hours in the presence of Nile red. The cells were washed and resuspended in PBS.
Our Construct
- We then proceeded to test out plasmid in the plate reader.
- Cells were grown for 24 hours with both enzymes induced and in the presence of Nile red. The cells were washed and resuspended in PBS. The readings were normalized for optical density.

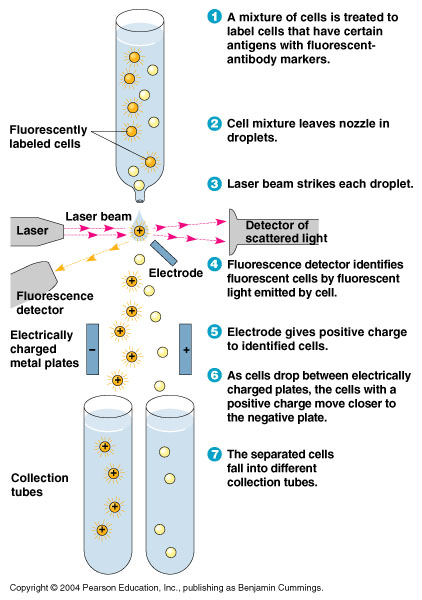
FACS Sorting
- In order to quickly screen the large diversity we planed to create using MAGE, we wanted to employ Fluorescence-activated cell sorting (FACS)
- FACS could sort cells based on the Nile red fluorescence, thus indicating those cells that have produced larger quantities of PLA
- Nile red has an emission maximum at 598nm when bound to p(3HB) granules according to Spiekermann et al. 1998
- Thus we decided to use PE-Texas Red which has an emission maximum at 615nm in order pick the best colonies.
 "
"
