Team:NYMU-Taipei/Project/Inhibition/Prohibition
From 2013.igem.org


Contents |
Prohibiting Sprouting
Introduction
In order to stop the invasion of Nosema ceranae, the first step we take is to fully understand its life cycle which completes by bees. The picture beside indicates the relation betweenNosema ceranae stage and the place it grows. Through this picture, we can plan different ways to block the invasion of N. ceranae at each stage and also analyze the advantages and drawbacks of each ways, as well as to decide which stage is the best and most attainable for us to weaken the transmission ability of Nosema ceranae.
Background
Stop it at the very begining!!!
After N. ceranae invades epithelial cells of a bee's midgut, it will soon reproduce in two days and new generation appears. Since its lifecycle is so short that there is no time for biological methods to react and inhibit the development of the pathogen, we choose to inhibit it before entering bees' epithelial cells, which will be before N. ceranae’s germination. When N. ceranae's spore moves to bees' midgut, it will start to germinate and develop a structure called polar filament. This structure will then bind to epithelial cells of bees' midgut and poke a hole at the membrane of an epithelial cell, causing the bee eventually dead. In order to inhibit polar filament's development, we find out that there is a protein called PTP1, a protein on the surface of the polar filament, is highly relevant to the binding of the polar filament and epithelial cells. It is also highly believed that obstructing the production of PTP1 can stop the invasion of N. ceranae into the epithelial cell of the bee's midgut. So we decide to suppress the growth of the polar filament by obstruct the production of PTP1. In the research that is done by other scientist, they have found out that in the process of PTP1 formation a mannose polymer will bind to the protein. We initially decided to use mannosidase as our weapon to stop the invasion, however, we have considered the efficiency of how fast the enzyme can degrade the polymer, also due to the difficulty to test its function, we changed our weapon into FimH, which is a mannose binding protein. In this way, at the appearance of the polar filament our protein can immediately block PTP1 and stop the invasion of N. ceranae.
FimH
FimH protein, a protein expressed in Escherichia coli K-12 substrain MG1655, has a mannose-binding domain from the 22nd to 179th amino acid. FimH protein is originally located on the tip of the fimbria of the E. coli, which will adhere to the mannose on the host's cell membrane, allowing the bacteria to colonize various host tissues. In our project we managed to use it to bind the mannose polymer, which serves as a sticky end to bind the host cell, on top of the PTP1, in order to stop the adhesion between the polar filament and the epithelial cell of the bee's midgut.
Circuit Design
Experiment
To check whether FimH really works in our Bee. coli, we fused RFP and FimH protein mannose-binding domain([http://parts.igem.org/Part:BBa_K1104101| BBa_K1104101]), so that when FimH binds to the mannose polymer, we can easily observe through seeing the red fluorescence.We will call this fusion protein RFP-FimH on the following text.
Circuit Design
Through blunt-end ligation, we insert the 22nd to 179th amino acid of FimH protein gene in front the stop codon of the RFP coding sequence, in order to form a protein fusion. The promoter for this circuit is the LacI regulated promoter.We use this promoter as a constitutive promoter, since there is no LacI protein expressed in the bacteria. The picture on the right is the circuit design for our experiment.([http://parts.igem.org/Part:BBa_K1104102| BBa_K1104102])
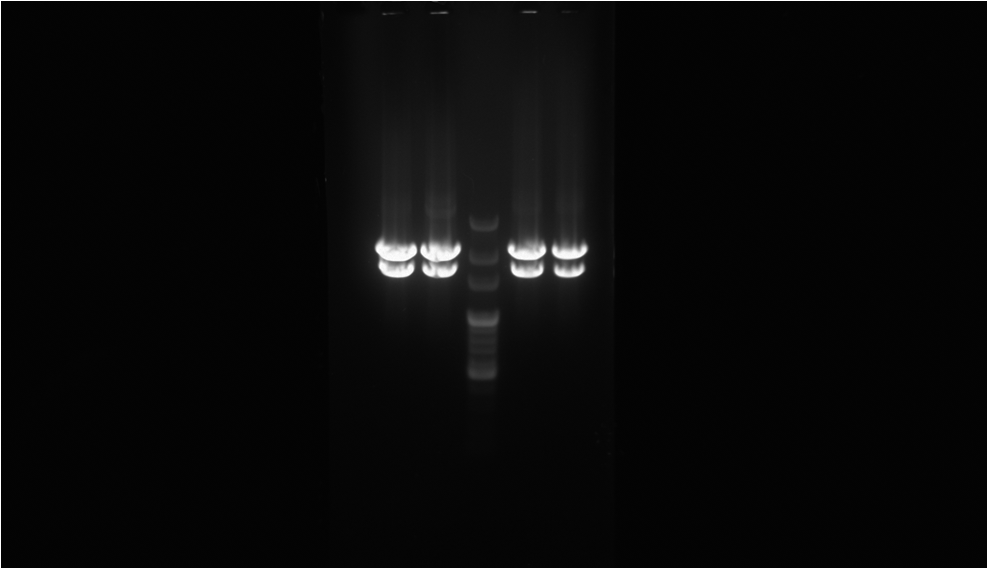
To proof that the protein fusion actually express in the E. coli we have done digestion on XbaI and PstI cutting site to proof that the length of our biobrick is right. So there is only the coding sequence of RFP-FimH instead of RFP only. The picture on the right is the result of the electrophoresis after the digestion. Secondly, through observing the fluorescence emitted by the E. coli we can be sure that we have produced the protein that we want, which is the RFP-FimH fusion protein. The result is as the picture on the left.
RFP-FimH functional test
After cloning RFP-FimH gene into E. coli, it is a must to examine whether our part is properly combined and well-functioned. Therefore we decide to extract RFP-FimH fusion protein from the cytoplasm of the E. coli and test its function by observing whether it will bind to the mannose. We choose E. coli to be the material that carries the mannose to conduct the functional test.
Experimental process is listed below
1.Sonicate the E. coli that can produce RFP-FimH fusion protein
2.Centrifuge under 13200rpm for 1 min. and keep the supernatant
3.Cultivate Escherichia coli K-12 substr MG1655 for 2 hours
4.Take 100λ of liquid
5.Centrifuge under 13200rpm for 2 min.
6.Discard the supernatant and add in 100λ of the protein extraction
7.Fully mix the E. coli with the protein extraction
8.Put the mixture under 4℃ for 30 min.
9.Centrifuge the mixture under 13200rpm for 1.5 min.
10.Observe the pallet under the fluorescence microscope and see if there is fluorescence emitted
experiment group:
| control group | experimental group | |
| protein added | RFP | RFP-FimH |
Experiment data
We anticipate that the group adding the RFP-FimH will express red fluorescence and the group adding RFP will not express red fluorescence in the pallet. Since the protein with FimH will bind to the mannose on the E. coli, so it will express red fluorescence. The picture on the right shows that the group with FimH has a red fluorescence. Which means FimH that we cloned really works, and can surely block the mannose polymer on the PTP1 and stop the invasion of N. ceranae into the epithelial cell.
Reference
1. EcoCyc: Encyclopedia of Escherichia coli K-12 Genes and Metabolism [http://ecocyc.org/ECOLI/NEW-IMAGE?type=ENZYME&object=EG10315-MONOMER#/ FimH]
2. XU, Y., & WEISS, L. (August 01, 2005). The microsporidian polar tube: A highly specialised invasion organelle. International Journal for Parasitology, 35, 9, 941-953.
3. Xu, Yanji, Takvorian, Peter M., Cali, Ann, Orr, George, & Weiss, Louis M. (n.d.). Glycosylation of the Major Polar Tube Protein of Encephalitozoon hellem, a Microsporidian Parasite That Infects Humans. American Society for Microbiology.
4. Xu, Yanji, Takvorian, Peter M., Cali, Ann, Orr, George, & Weiss, Louis M. (n.d.). Glycosylation of the Major Polar Tube Protein of Encephalitozoon hellem, a Microsporidian Parasite That Infects Humans. American Society for Microbiology.
 "
"