From 2013.igem.org
Transformation results
Gel Electrophoresis
- The double digest (pSB1C3) from yesterday was run on a 1% gel.
- This was done in order to confirm that the digest worked and also to purify the pSB1C3 vector.
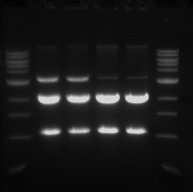
- Sample 1.1 - track 2
- Sample 1.2 - track 3
- Sample 2.1 - track 4
- Sample 2.2 - track 5
- The 2 Kb bands are the pSB1C3 backbone
- The 1 Kb bands are the RFP biobrick
The two higher bands in tracks 2 and 3 are plasmids that were not digested.
Gel purification of the pSB1C3 backbone and RFP biobrick
- The Zymoclean Gel DNA recovery Kit was used to perform the purification and its protocol was followed.
- However the elution step was changed to 12ul of elution buffer, and this step was repeated.
Nanodrop of the samples
| Sample | Volume | Concentration ng/ul | 260/280 | 260/230
|
| pSB1C3 1 | 19.8 | 31.9 | 1.83 | 1.75
|
| pSB1C3 2 | 20 | 34 | 1.67 | 0.92
|
| RFP 1 | 20 | 28.8 | 1.57 | 0.71
|
| RFP 2 | 18.8 | 22.2 | 1.79 | 1.84
|
Double digest of TOD genes PCR products
Samples A (TodX), C (TodF) and E (ToBG) were digested with the enzymes XbaI and SpeI.
| Samples | A | C | E
|
| Volume (ul) | 20 | 23.5 | 21
|
| SpeI (ul) | 1 | 1 | 1
|
| XbaI | 0.5 | 0.5 | 0.5
|
| Cutsmart Buffer | 6 | 6 | 6
|
| 5mTris Hcl (ul) | 32.5 | 29 | 31.5
|
- The samples were incubated at 37C for 90 minutes
- Heat kill the enzymes by incubating the samples at 80C for 20 minutes
Ligation of the TOD genes (X, F and ToBG) to the pSB1C3 backbone
The pGEM-T Vector System was used, However changes were made to the protocol.
The promega biomath caculator (www.promega.com/biomath) was used to work out the ratio of insert to vector.
The DNA concentration of the TOD genes can be found at Lab work 28/09/2013.
The DNA concentration of the pSB1C3 and the RFP biobrick can be found above.
The amount of vector DNA was added at 50ng.
Protocol
| Samples | A (TodX) | C (TodF) | E (ToBG) | RFP-sample 2 (positive control) | Background control
|
| 2X Rapid ligation buffer | 5 | 5 | 5 | 5 | 5
|
| vector (pSB1C3-sample1) | 1.6 | 1.6 | 1.6 | 1.6 | 1.6
|
| T4 DNA ligase | 1 | 1 | 1 | 1 | 1
|
| PCR product | 2.3 | 1.4 | 3.1 | 3.6 | -
|
| Deionized water for the final volume of 12 ul | 2.1 | 3 | 1.3 | 0.8 | 4.4
|
 "
"
