Decontaminating XL10 Gold
Main Page

Contents |
Decontaminating XL10 Gold
Purpose
To remake the XL10 Gold E. coli to remove the Ampicillin resistance that has contaminated the original.
Background
XL10 Gold competent cells are one of our top preferences when it comes to cells to use to transform DNA. This is due to the high transformation efficiency of the strain (~1x10^9). However, we noticed that our XL10 Gold Competent cells had been contaminated with some sort of Ampicillin resistance when there were a significant amount of colonies growing on our negative control Ampicillin plates.
Starting Materials
- Contaminated Competent XL 10 Gold E. coli cells
Design
The goal of this project is to eliminate or, at least, reduce the ampicillin contamination in the XL 10 Gold competent cells. These cells have a high transformation efficiency and therefore, is a competent cell that we use quite often. XL 10 Gold colonies growing on the negative control plate has been observed. This means that they have some intrinsic ampicillin resistance. To try tackle this problem, several steps are taken. Here is the design of the project:
- Streak the frozen XL 10 Gold stock onto a chloramphenicol (XL 10 Gold has intrinsic CM resistance) plate.
- Pick 3 colonies and grow then in chloramphenicol and LB.
- Plate 125uL from these 3 media cultures onto ampicillin plates
- Check which plate has the least amount of growth
- Make a new frozen stock using the media culture that yielded the least amount of colonies on the ampicillin plate
Hopefully this new frozen stock is not contaminated with ampicillin.
Finally, the competent cell process needs to be done in order to make the XL 10 Gold cells competent again. Using the media culture that yielded the least amount of colonies, competent cells protocol is performed, and a transformation is done using the competent cells. Hopefully there will be no growth on the ampicillin plate (negative control) this time.
Results
June 26th, 2013
Streaked a chloramphenicol using the frozen stock of XL10 Gold cells. Incubated at 37 degrees overnight.
June 27th, 2013
Picked 3 colonies and made 3 media cultures of XL10 Gold. Incubated at 37 degrees overnight.
June 28th, 2013
Plated the 3 media cultures on ampicillin plates.
June 29th, 2013
Plate made from media culture 1:
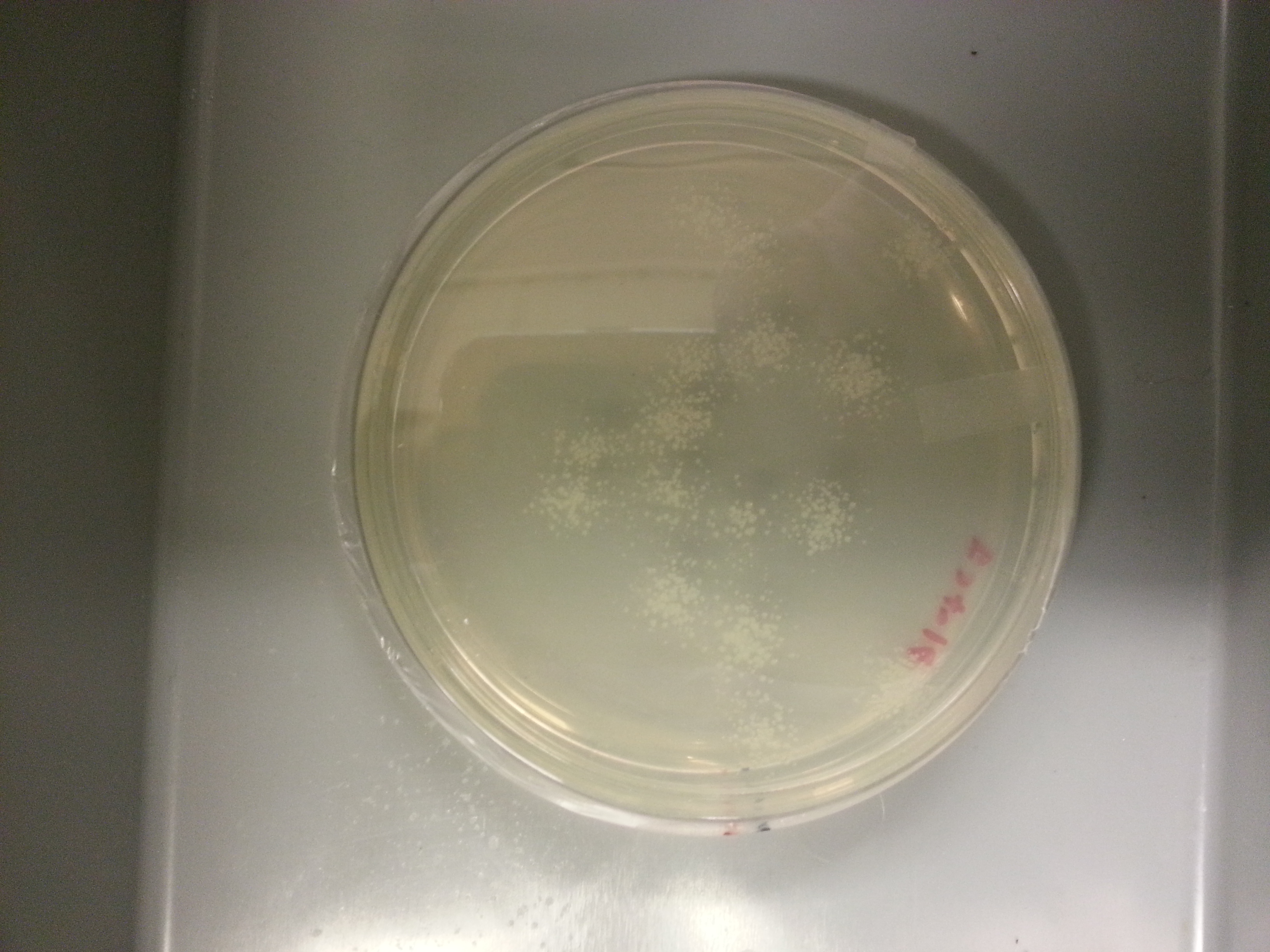
Plate made from media culture 2:
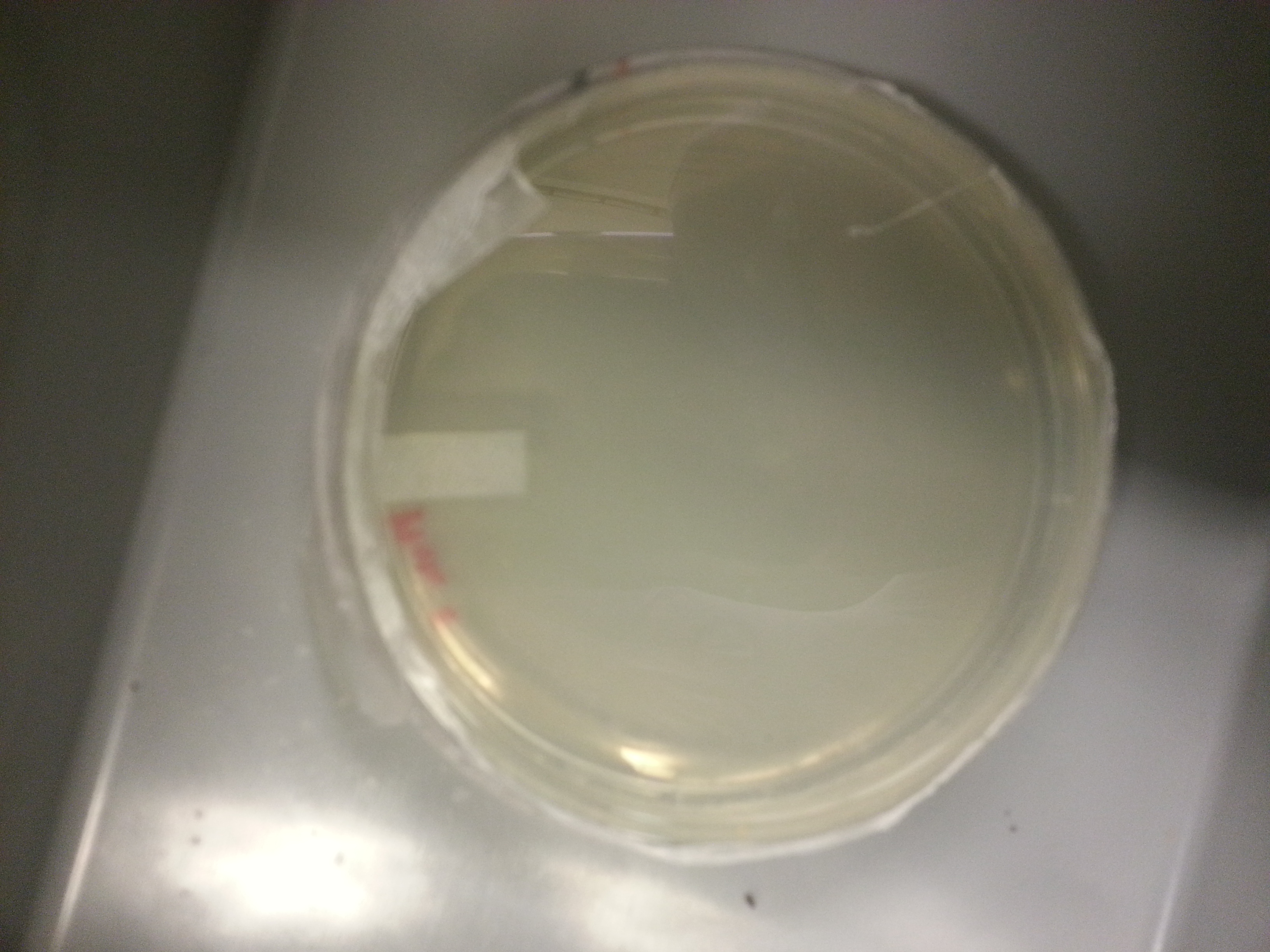
Plate made from media culture 3:
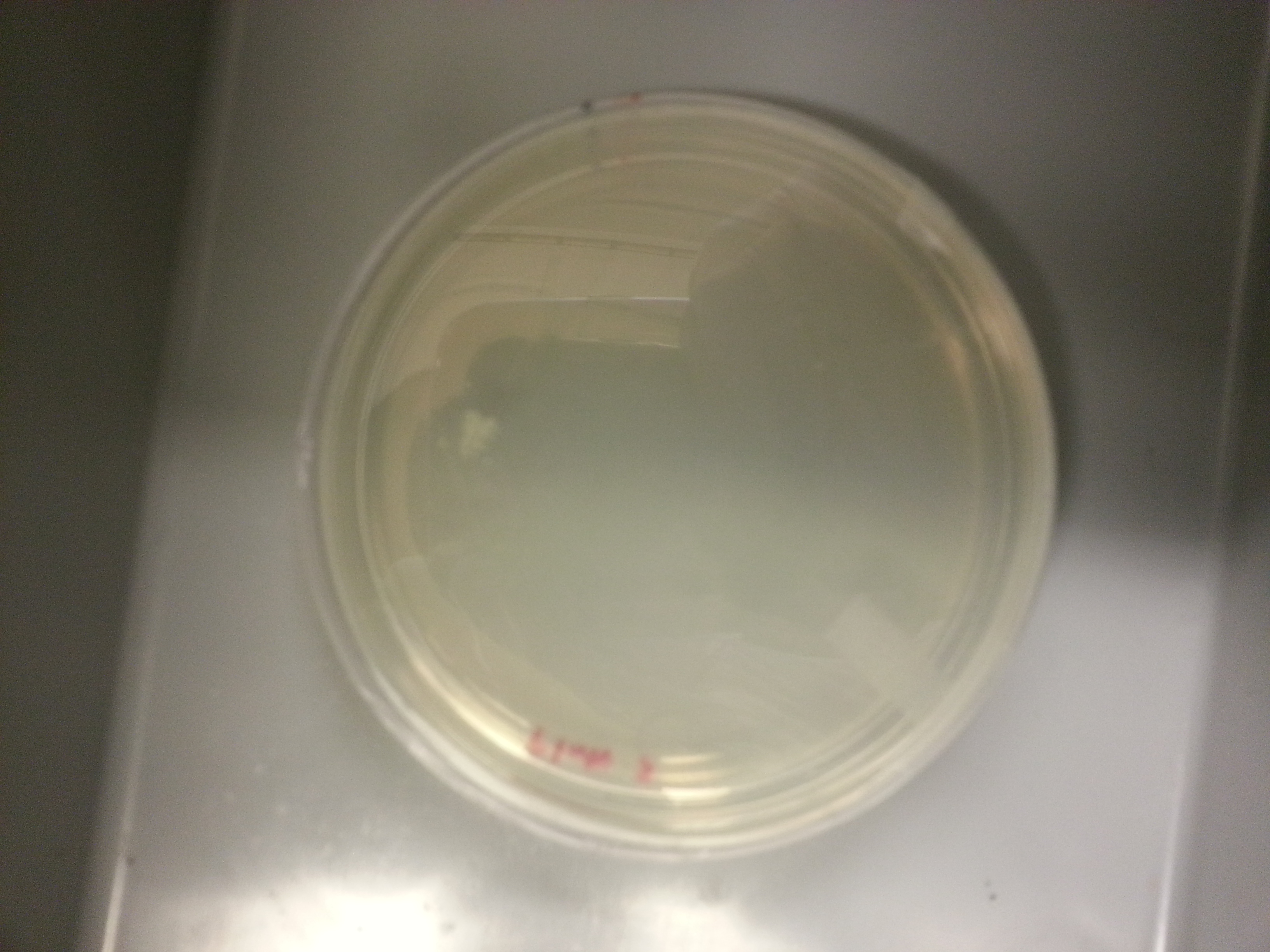
Since plate 2 had the least amount of colonies, an agar stab and frozen stock is made using media culture 2.
June 30th, 2013
2 flasks are filled with 100mL of SOB media. One flask is inoculated with 1mL of XL10 Gold culture and the other flask is inoculated with 100uL of the culture @ 10PM. The flasks are placed in the 18 degree shaker.
July 1st, 2013
9AM 1mL inoculated flask: 0.042 100uL inoculated flask: 0.000
1PM 1mL inoculated flask: 0.120
July 2nd, 2013
10AM 1mL inoculated flask: 0.226
2:30PM 1mL inoculated flask: 0.562
Competent cell preparation can continue according to Competent Cells Protocol.
Competent cells were made and aliquoted at 6PM.
July 3rd, 2013
Competent cells are transformed using the PUC18 vector. The plasmid is diluted to 1000, 100, and 10 picograms per microliter.
July 5th, 2013
Very high transformation efficiency, but too many colonies to count. Still lots of growth on the negative control plate. Pattern of growth is not uniform. This leads us to suspect that the ampicillin is destroyed in some parts of the plate.
July 6th, 2013
Transformation is redone using smaller concentrations to calculate the transformation efficiency. There is minimal growth on the negative control this time which leads us to believe that the contamination has been reduced. Transformation efficiency is 3.7x10^6 (37 colonies on plate with 10 picograms of DNA).
Conclusion
There is minimal growth on the negative control this time which leads us to believe that the contamination has been reduced. Transformation efficiency is 3.7x10^6 (37 colonies on plate with 10 picograms of DNA).
 "
"
