From 2013.igem.org
10-07-2013
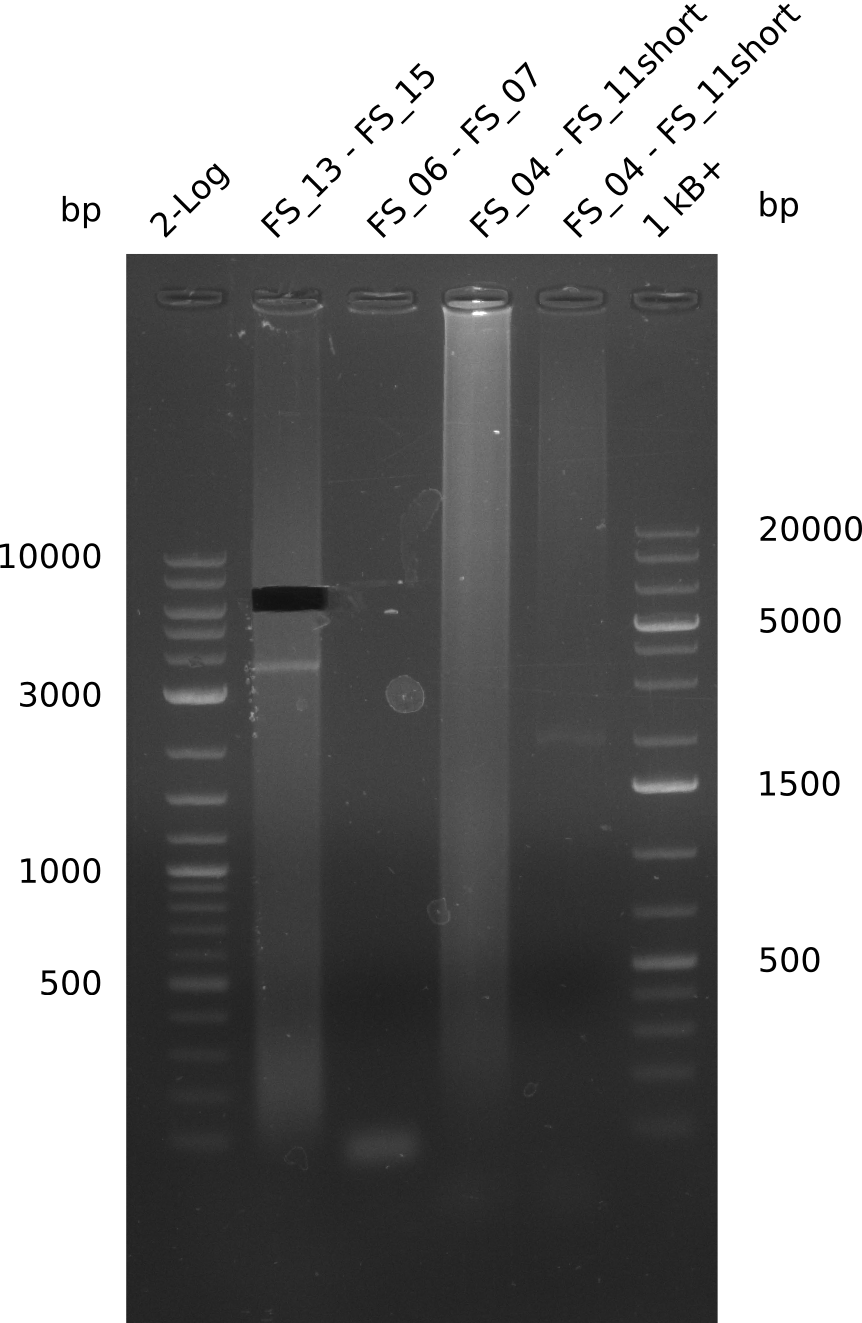
Amplification I from FS_06 to FS_07; 5.2 kb
PCR for amplification of DelOP, DelFG and DelG; +=with DMSO, -=without DMSO, L=both primers are the long primers, S= one Primer is one of the short primers; lane1=Marker, lane2=delOP(04.07,L+), lane3=DelOP(10.07,L+), lane4=DelOP(10.07,L-), lane5=DelOP(10.07,S+), lane6=DelOP(10.07,S-), lane7=DelFG(10.07,+), lane8=DelFG(10.07,-), lane9=DelOP(09.07,S+), lane10=DelG(09.07,S+); expected amplicon sizes: DelOP=2.5kbp, Del DelFG=ca 5kbp, DelG=6.4kbp; run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 1
|
| FS_07: (1/10) | 1
|
| Phusion Master Mix | 10
|
| DMSO | 1/-
|
| dd H2O | 6/7
|
- Conditions
| Biorad MyCycler
|
| Cycles-PCR | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 66 ↓ 0.5 | 5
|
| 72 | 2:10 min
|
| 18 | 98 | 1
|
| 63 | 5
|
| 72 | 2:10 min
|
| 1 | 72 | 10 min
|
| 1 | 4 | inf
|
Results:
- Amplification did not work, not product is visible
- PCR will be repeated with lower annealing temperature to increase primer binding
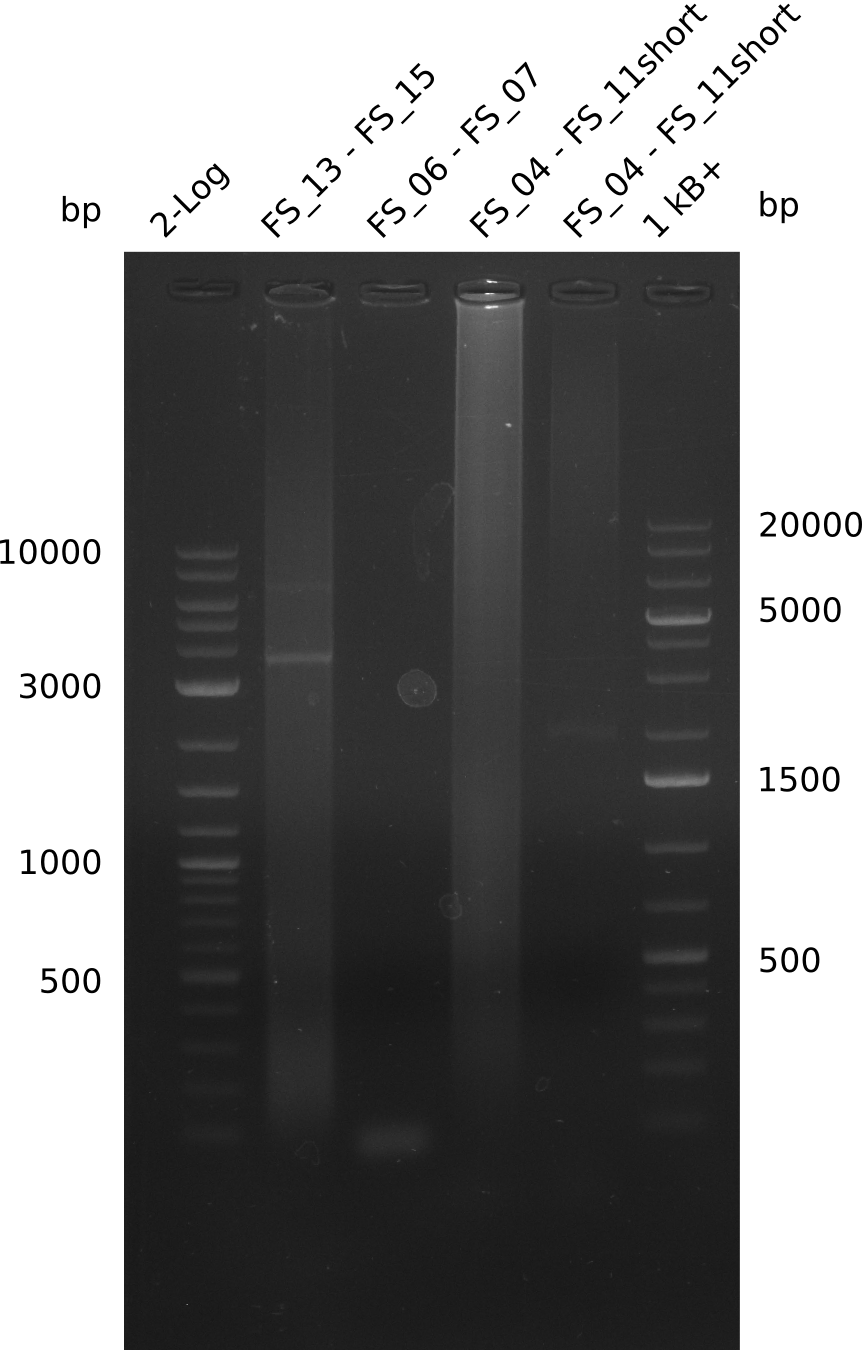
Amplification II from FS_06 to FS_07; 5.2 kb
PCR for amplification of DelFG II; lane1= Marker, lane2= touchdown with DMSO, lane3= touchdown without DMSO, lane4=constant anealing with DMSO, lane5= constant without DMSO ; expected amplicon size: DelFG=ca.5kbp; run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 1
|
| FS_07: (1/10) | 1
|
| Phusion Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 6
|
- Conditions
| Biorad MyCycler
|
| Cycles-PCR | temperature [°C] | Time [s]
|
| 1 | 98 | 5
|
| 30 | 98 | 1
|
| 58 | 5
|
| 72 | 2:30 min
|
| 1 | 72 | 10 min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work
- PCR will be repeated with higher annealing temperature as reaction might not have worked due to secondary structures of primers
Amplification III from FS_06 to FS_07; 5.2 kb
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 1
|
| FS_07: (1/10) | 1
|
| Phusion Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 6
|
- Conditions
| Biorad MyCycler
|
| Cycles-PCR | temperature [°C] | Time [s]
|
| 1 | 98 | 5
|
| 12 | 98 | 1
|
| 60 ↓ 0.5 | 5
|
| 72 | 2:30 min
|
| 18 | 98 | 1
|
| 58 | 5
|
| 72 | 2:30 min
|
| 1 | 72 | 10 min
|
| 1 | 12 | inf
|
11-07-2013
PCR for amplification of DelLP, DelFG and DelEG; lane1=Ladder log2, lane2=delLP(11.07), lane3=DelFG(11.07), lane4=DelEG (Phusion flash, 11.07), lane5=DelEG (Phusion II,11.07), lane6=1kb ladder plus; DelLP=6.4kbp, DelFG=5.3kbp, DelEG=16.4kbp; run at 100 V, 0.8 % gel (TAE)
Amplification from FS_06 to FS_07; 5.2 kb
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 1
|
| FS_07: (1/10) | 1
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 6
|
- Conditions
| Biorad MyCycler
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 68 ↓ 0.5 | 5
|
| 72 | 2:30
|
| 18 | 98 | 1
|
| 66 | 5
|
| 72 | 2:30
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work
- other primers will be designed in order to amplify the desired sequence from D. acidovorans
12-07-2013
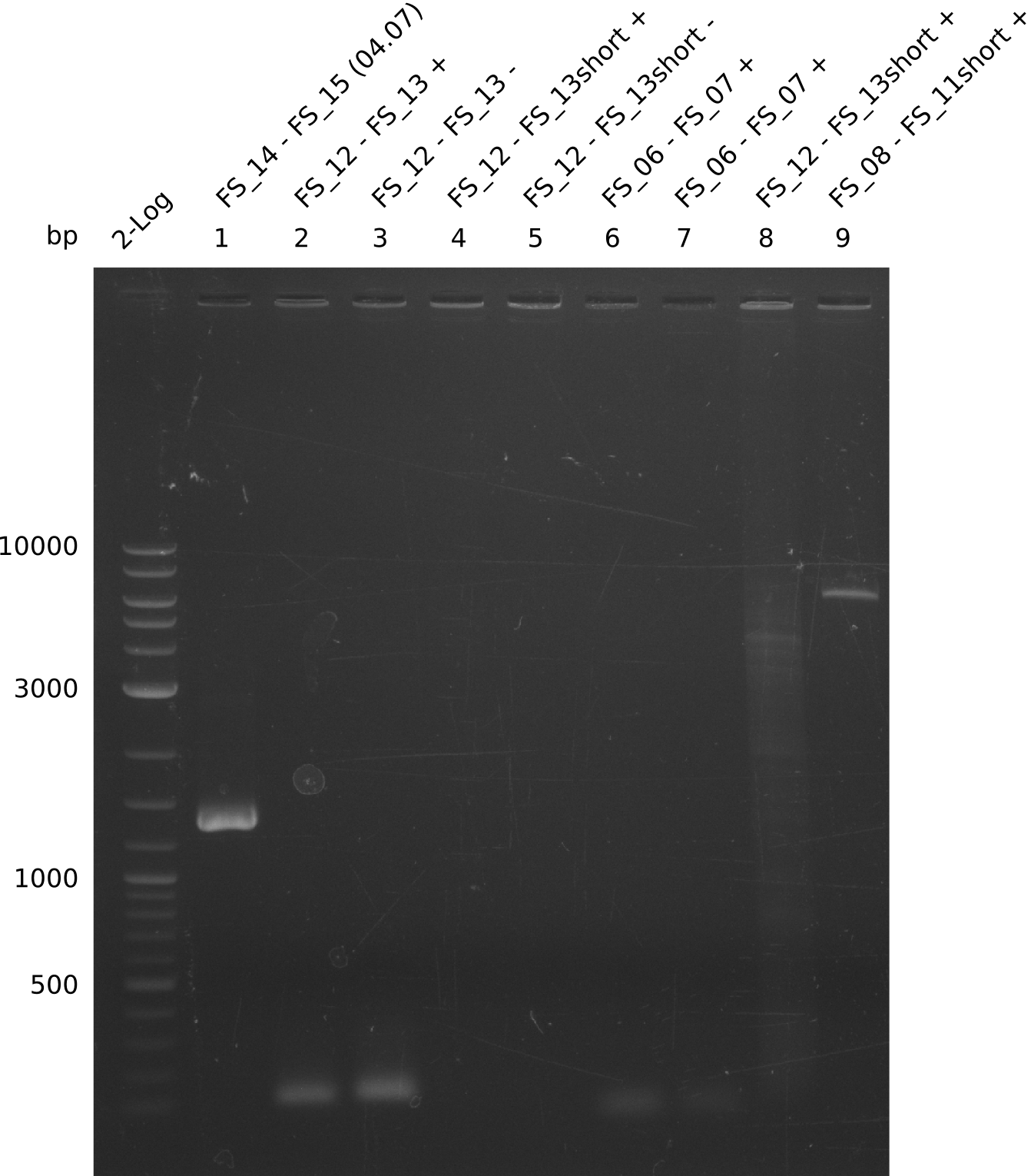
Amplification from FS_06 to FS_11(s); 11.6 kb
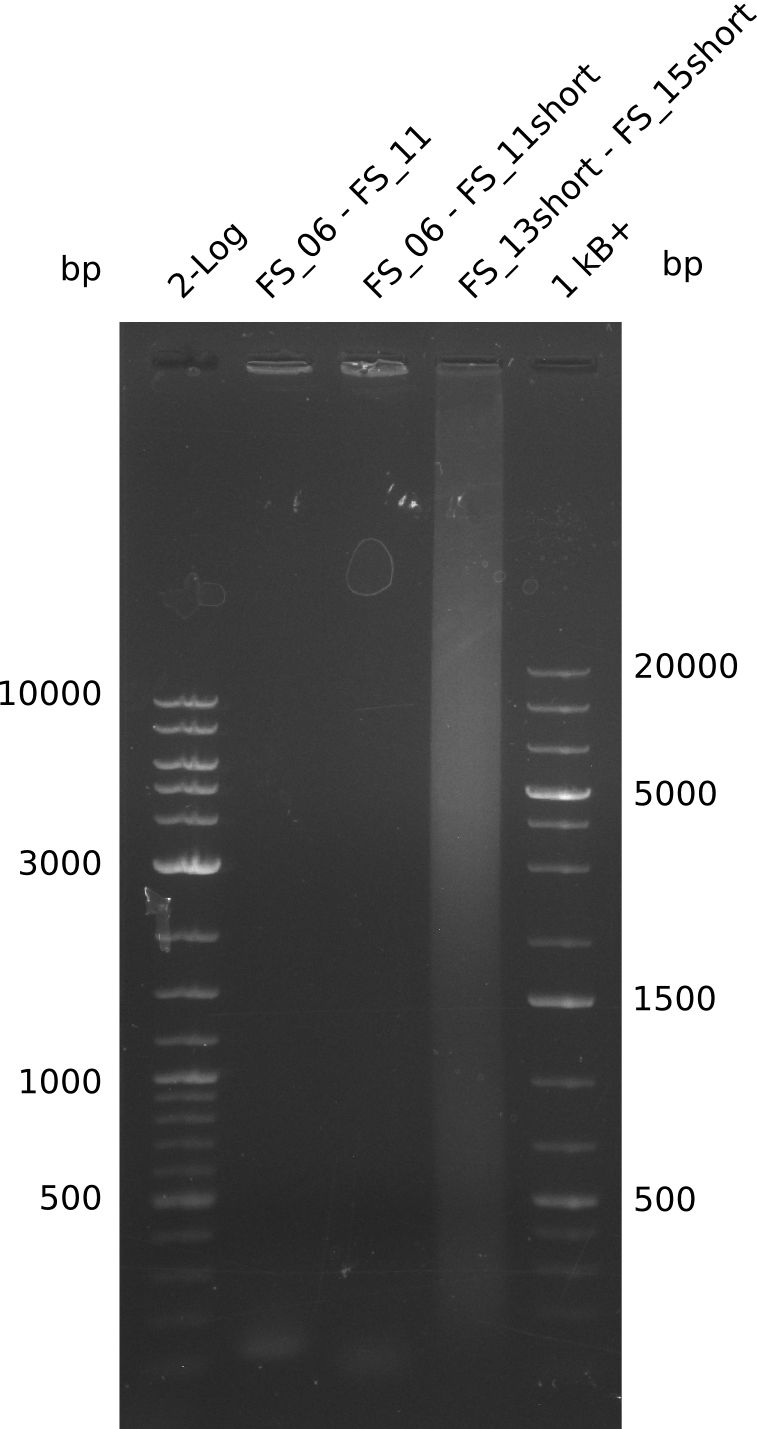
PCR for amplification of DelFG (12.07;FS6+FS11) and re-amplification of DelLP(12.07;FS13short+FS15short); lane1=Ladder log2, lane2=DelFG(12.07;FS6+FS11long), lane3=DelFG(12.07;FS6+FS11short), lane4=DelLP, lane5=1kb ladder plus; DelLP=6.4kbp, DelEG=11.6 kbp; run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 2
|
| FS_11 (long or short): (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
Attention: 4 cycles were accidently carried out with an elongation time of 3:00 min
| Biorad MyCycler
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 11 | 98 | 1
|
| 73 ↓ 0.5 | 5
|
| 72 | 5:00
|
| 19 | 98 | 1
|
| 68 | 5
|
| 72 | 5:00
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work, neither with the short nor with the long version of FS_11, consequently PCR will be repeated with different primers
13-07-2013
Amplification from FS_06 to FS_09; 8.5 kb
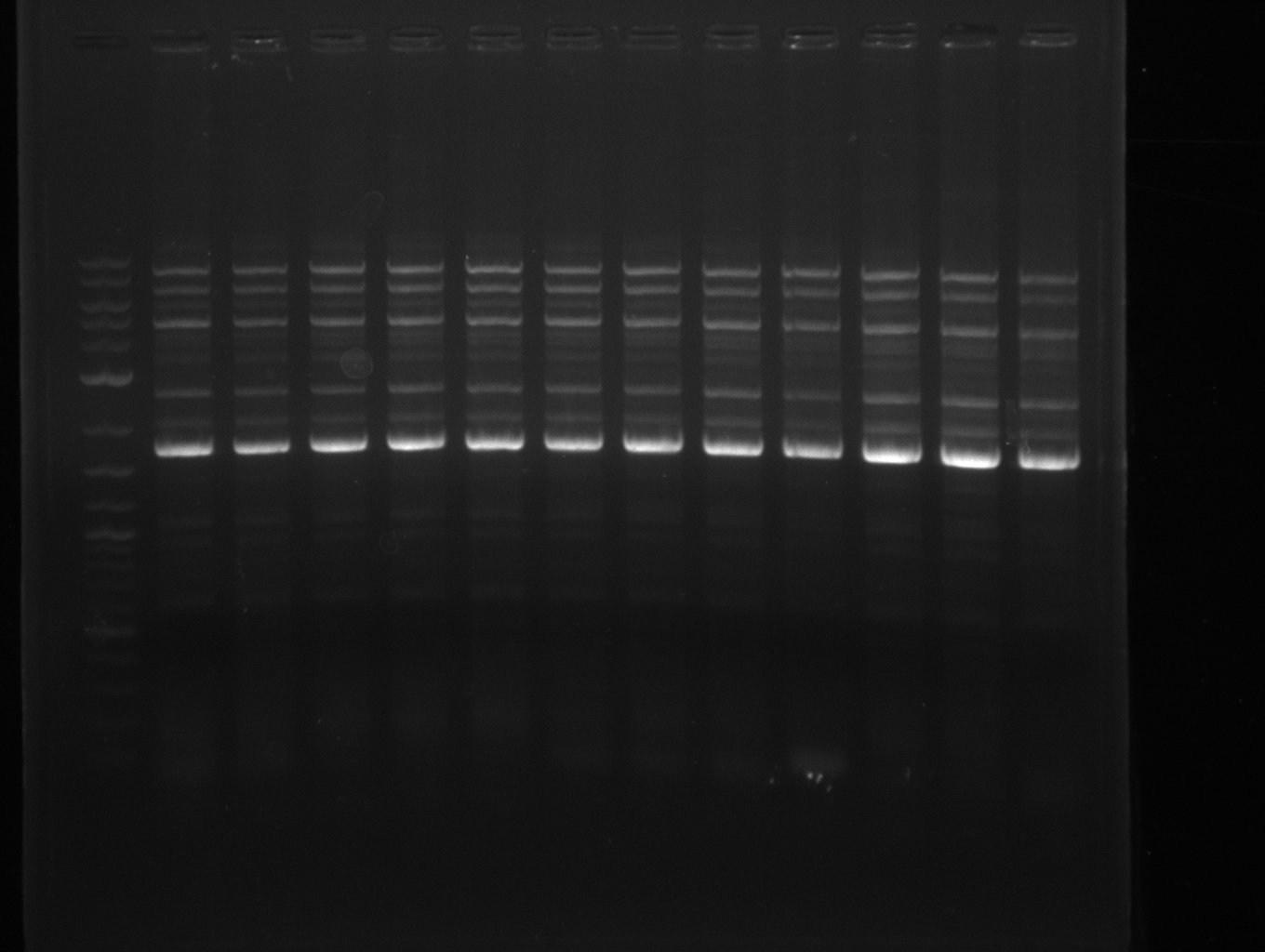
PCR for amplification of DelFG (13.07; FS6-FS9); run at 100 V, 0.8 % gel (TAE)
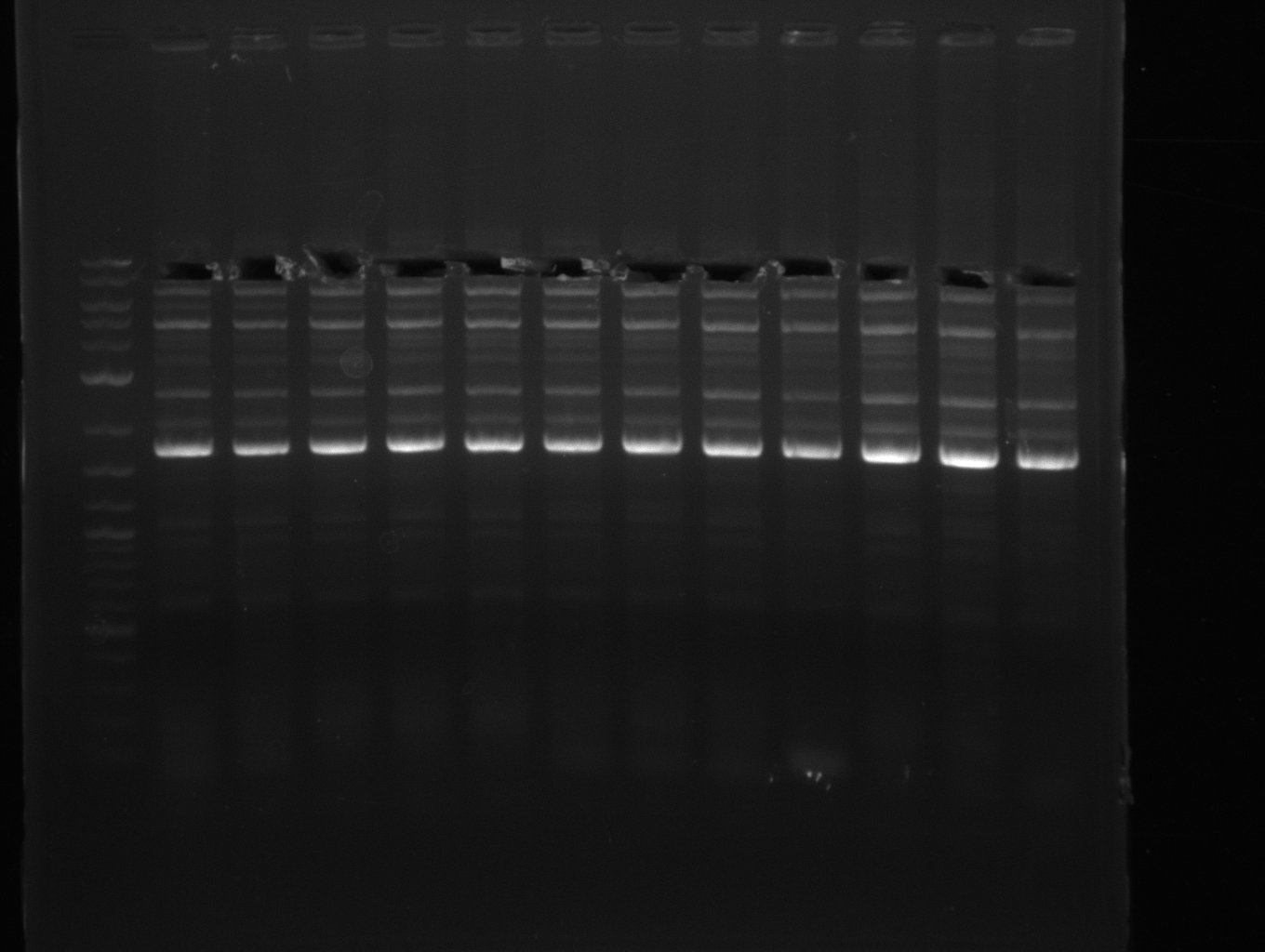
PCR for amplification of DelFG (13.07; FS6-FS9) after excision
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 2
|
| FS_09: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
a gradient PCR combined with touchdown was carried out.
12 wells, gradient in the annealing temperature from 74°C - 66°C,
resulting in a gradient of 73°C - 65°C in the constant program
| Biorad MyCycler
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 74 ↓ 0.5 to 65 ↓ 0.5 | 5
|
| 72 | 3:00 min
|
| 18 | 98 | 1
|
| 73 to 65 | 5
|
| 72 | 3:00 min
|
| 1 | 72 | 10 min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG worked, though several other bands occured, indicating low primer specifity
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- final concenctration after QIAquick Nucleotide Removal Kit: 5ng/µl in 18µl
14-07-2013
Re-Amplification from FS_06 to FS_09; 8.5 kb; 13-07-2013)
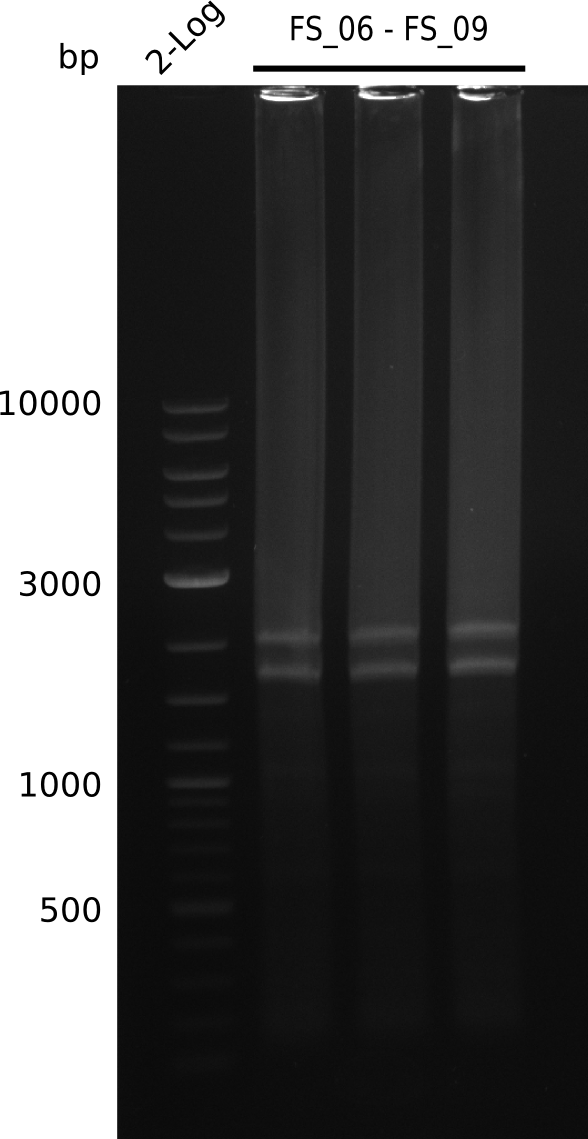
PCR for amplification of DelFG (14.07) run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| Template of gel extraction (13-07-2013) | 1
|
| FS_06: (1/10) | 5
|
| FS_09: (1/10) | 5
|
| Phusion flash Master Mix | 25
|
| DMSO | 2.5
|
| dd H2O | 11.5
|
- Conditions
| Biorad MyCycler
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 71 ↓ 0.5 | 5
|
| 72 | 3:00 min
|
| 18 | 98 | 1
|
| 70 | 5
|
| 72 | 3:00 min
|
| 1 | 72 | 10 min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work, no product was detectable

 "
"