From 2013.igem.org
22-07-2013
Amplification from FS_20/FS_21 to FS_09; 8.5 kb
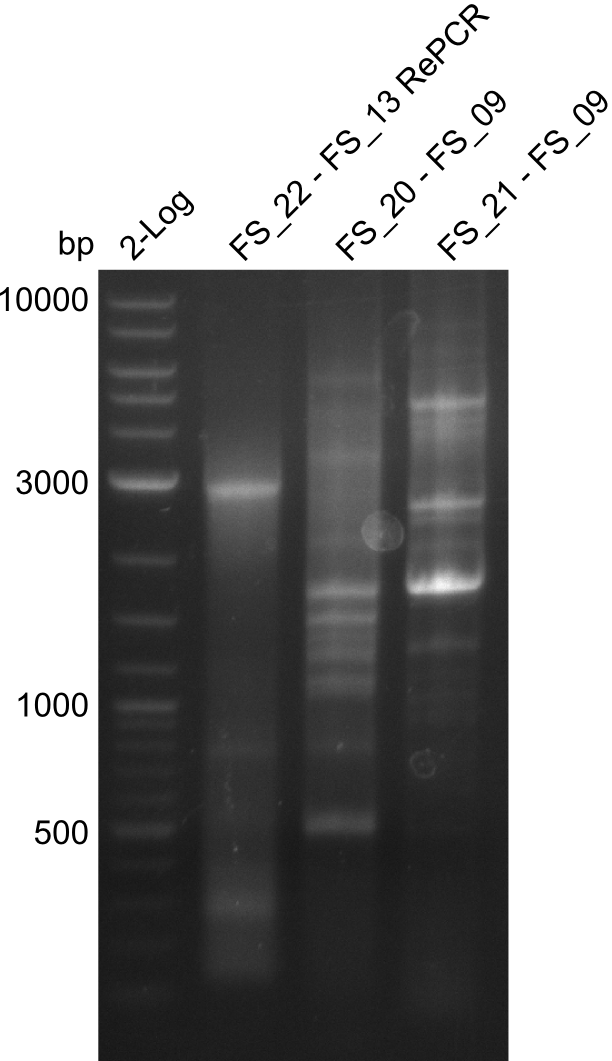
Re-PCR of DelOP (22-13long), Amplification of DelFG (20-9 and 21-9) (22.07); run at 100 V, 0.8 % gel (TAE)
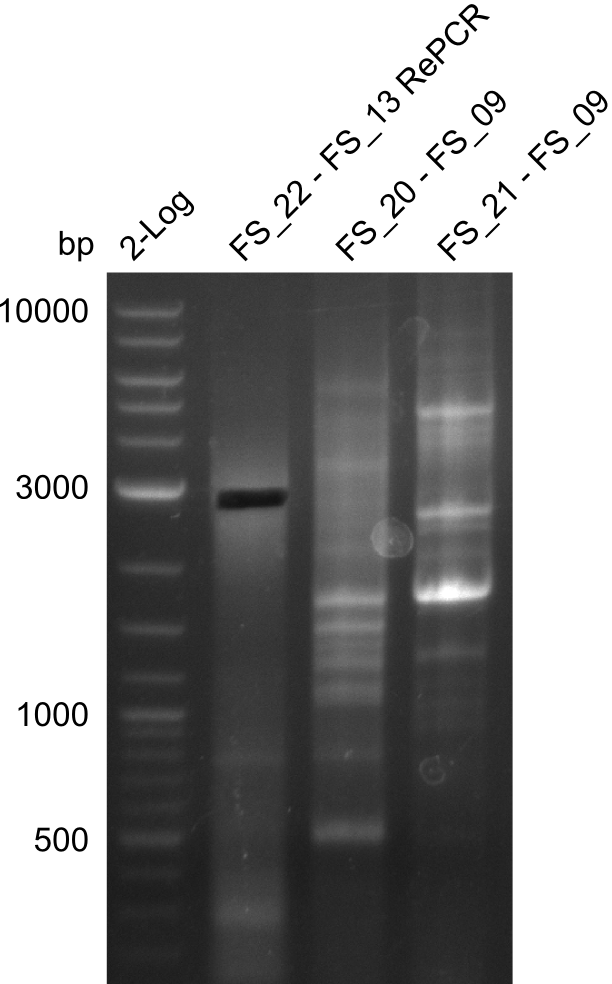
Re-PCR of DelOP (22-13long), Amplification of DelFG (20-9 and 21-9) (22.07) cut; run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20/FS_21: (1/10) | 2
|
| FS_09: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 65 ↓ 0.5 | 5
|
| 72 | 2:20
|
| 18 | 98 | 1
|
| 63 | 5
|
| 72 | 2:20
|
| 1 | 72 | 10 min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work
- other primers/combinations of primers will be used
- somehow getting annoyed by this part of D. Acidovorans
Amplification from FS_20/FS_21 to FS_11_short; 11.6 kb

Amplification of DelFG (22.07); run at 100 V, 0.8 % gel (TAE)
4x 20µl (70 touchdown, 65 touchdown)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20 or FS_21: (1/10) | 2
|
| FS_11_short: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions I
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 5
|
| 12 | 98 | 1
|
| 70 ↓ 0.5 | 5
|
| 72 | 3:40
|
| 18 | 98 | 1
|
| 68 | 5
|
| 72 | 3:40
|
| 1 | 72 | 13min
|
| 1 | 12 | inf
|
- Conditions II of Del FG
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 5
|
| 12 | 98 | 1
|
| 65 ↓ 0.5 | 5
|
| 72 | 3:40
|
| 18 | 98 | 1
|
| 63 | 5
|
| 72 | 3:40
|
| 1 | 72 | 13min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work neither with a touchdown PCR starting at an annealing temperature of 65°C nor 70°C
Amplification from FS_20/FS_21 to FS_23; 11.6 kb

Amplification of DelFG (22.07); run at 100 V, 0.8 % gel (TAE)
4x 20µl (70 touchdown, 65 touchdown)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20 or FS_21: (1/10) | 2
|
| FS_23: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions I
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 5
|
| 12 | 98 | 1
|
| 70 ↓ 0.5 | 5
|
| 72 | 3:40
|
| 18 | 98 | 1
|
| 68 | 5
|
| 72 | 3:40
|
| 1 | 72 | 13min
|
| 1 | 12 | inf
|
- Conditions II
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 5
|
| 12 | 98 | 1
|
| 65 ↓ 0.5 | 5
|
| 72 | 3:40
|
| 18 | 98 | 1
|
| 63 | 5
|
| 72 | 3:40
|
| 1 | 72 | 13min
|
| 1 | 12 | inf
|
Results:
- Neither the amplification with the primers FS_20 to FS_23 or FS_21 to FS_23 did work. Another primer combination has to be tried.
24-07-2013
Amplification of DelFG (FS_06 to FS_07; 5.2 kb)
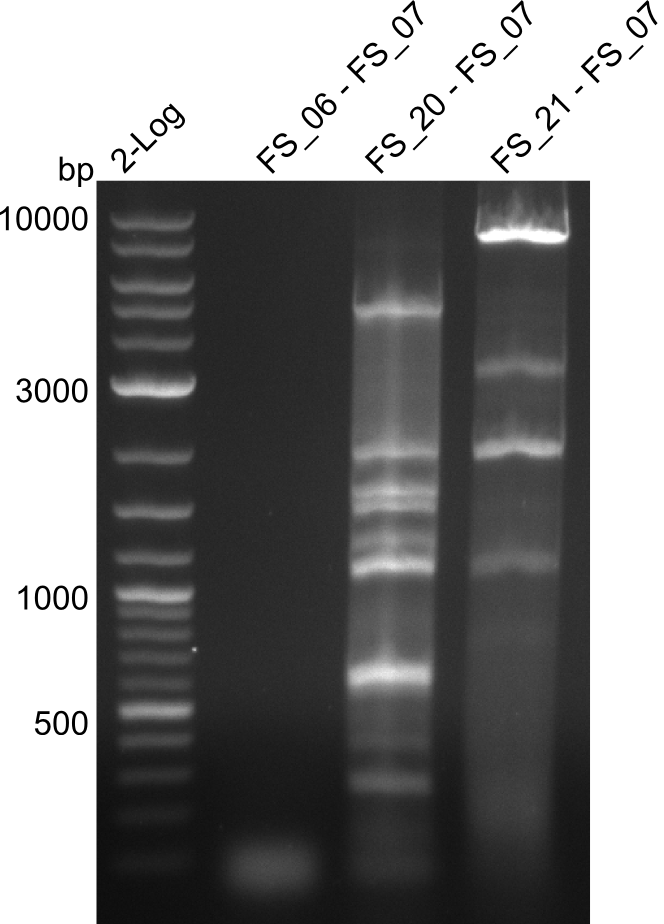
PCR of FG (FS_06 to FS_07) or (FS_20 to FS_07) or (FS_21 to FS_07) as indicated; run at 100 V, 0.8 % gel (TAE)
- Reaction I
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
Amplification from FS_20 to FS_07; 5.2 kb

PCR of FG (FS_06 to FS_07) or (FS_20 to FS_07) or (FS_21 to FS_07) as indicated; run at 100 V, 0.8 % gel (TAE)
- Reaction II
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
Amplification from FS_21 to FS_07; 5.2kb

PCR of FG (FS_06 to FS_07) or (FS_20 to FS_07) or (FS_21 to FS_07) as indicated; run at 100 V, 0.8 % gel (TAE)
- Reaction III
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_21: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
- Conditions for reactions I - III
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 68 ↓ 0.5 | 5
|
| 72 | 2:10
|
| 18 | 98 | 1
|
| 64 | 5
|
| 72 | 2:10
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- The amplification of FS_06 to FS_07 did not work. No bands were visible
- The amplification of FS_21 to FS_07 led to several bands, but none of these was the intended product
- The amplfication of FS_20 to FS_07 also led to several bands, one band at the right height was observed. Consequently the specificity of the PCR weill be increased by a higher annealing temperature
Amplification from FS_20 to FS_07; 5.2 kb
2x20µl (one with conditions I, other one with conditions II)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
- Conditions I
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 70 ↓ 0.5 | 5
|
| 72 | 2:10
|
| 18 | 98 | 1
|
| 68 | 5
|
| 72 | 2:10
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
- Conditions II
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 68 ↓ 0.5 | 5
|
| 72 | 2:10
|
| 18 | 98 | 1
|
| 66 | 5
|
| 72 | 2:10
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work, several bands occured, one of these had the size of the intended product but purity of the PCR was not sufficient for Gibson Assembly
- nethertheless bands were cut out and DNA purified using QIAquick Gel Extraction Kit for restriction digest
Amplification from FS_20 to FS_07; 5.2 kb
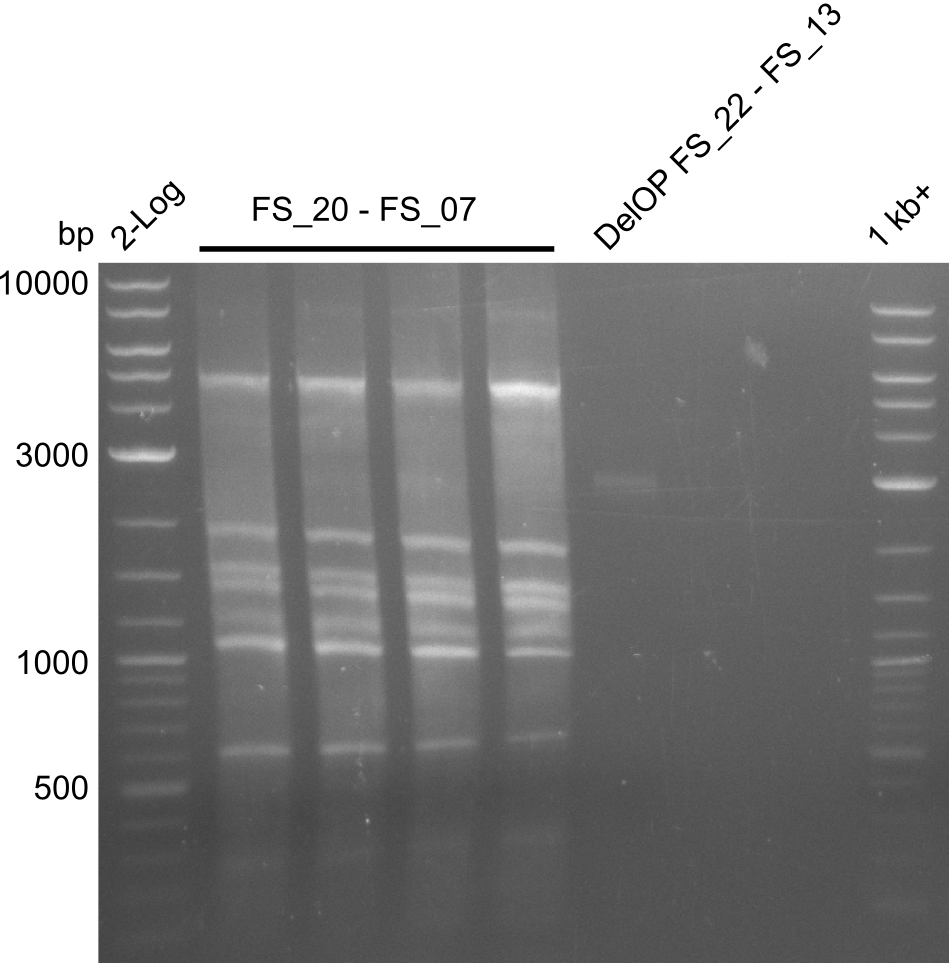
Amplification of DelFG II (FS20 to FS07; 24.07),; run at 100 V, 0.8 % gel (TAE)
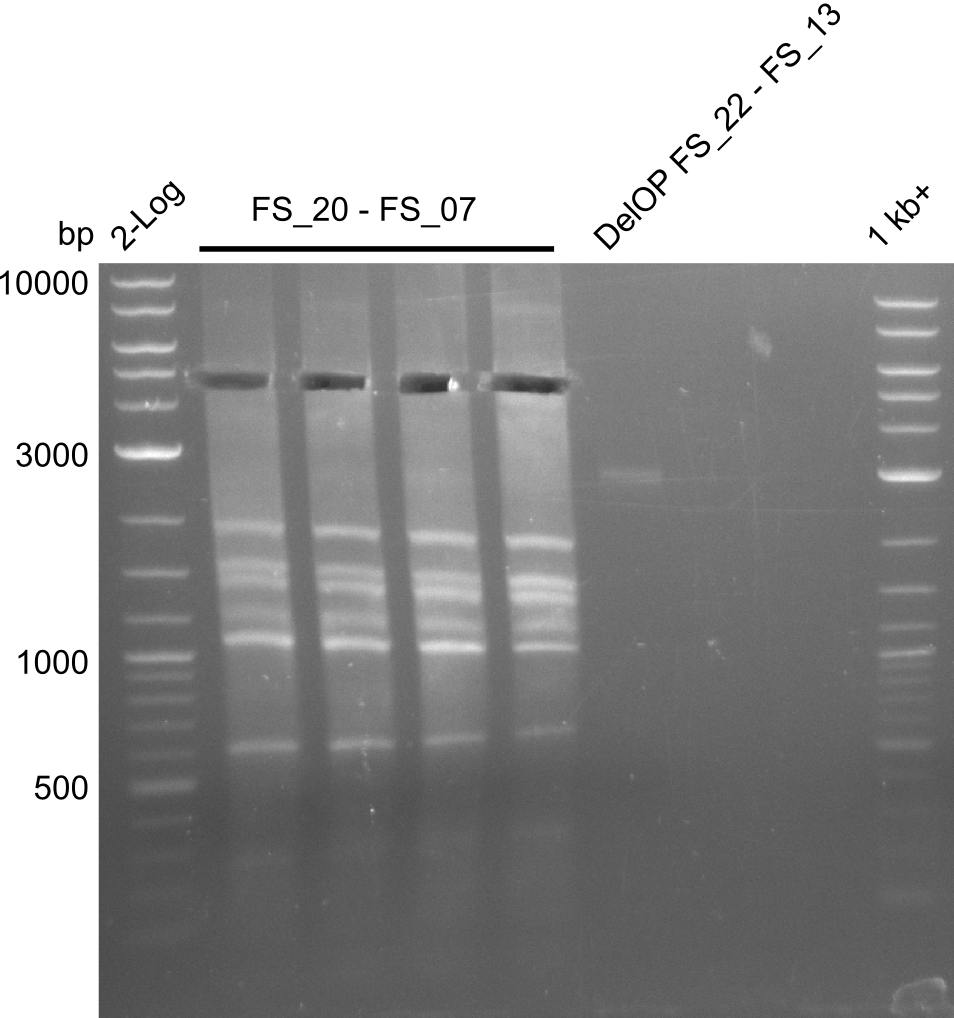
Amplification of DelFG II (FS20 to FS07; 24.07), ; run at 100 V, 0.8 % gel (TAE)
4x20µl
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
- Conditions
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 70 ↓ 0.5 | 5
|
| 72 | 2:10
|
| 18 | 98 | 1
|
| 68 | 5
|
| 72 | 2:10
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work, several bands occured, one of these had the size of the intended product but purity of the PCR was not sufficient for Gibson Assembly
- nethertheless bands were cut out and DNA purified using QIAquick Gel Extraction Kit for restriction digest
25-07-2013
Restriction digest of DelFG (FS_20 to FS_07; 5.2 kb; 23-07-2013 and 24-07-2013) with XmaI
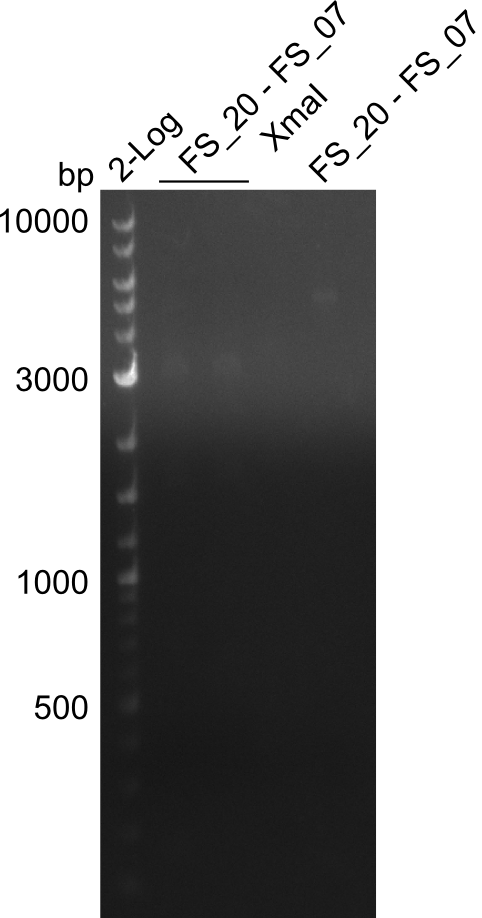
Restriction digest of Fragment FS_20 to FS_07 (23.07 and 24.07) with XmaI
The second lane is also a digest ; Expected size of digested fragments: 0.9kbp and 4.3kbp; run at 100 V, 0.8 % gel (TAE)
Incubation at 37°C for 45 min
Results:
- restriction digest of DelFG did not work, only very slight bands were visible
- digest will be repeated with higher amount of DNA and enzyme to improve analysis on the gel
26-07-2013
Amplification from FS_20 to FS_07; 5.2 kb
- Reaction I
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
- Conditions
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 70 | 5
|
| 72 | 2:10
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work, several bands occured, one of these had the size of the intended product but purity of the PCR was not sufficient for Gibson Assembly
- Annealing temperature will be increased further, to optimize primer specifity
Restriction digest of fragment from FS_20 to FS_07; 5.2 kb; 23-07-2013 and 23-07-2013) with ClaI
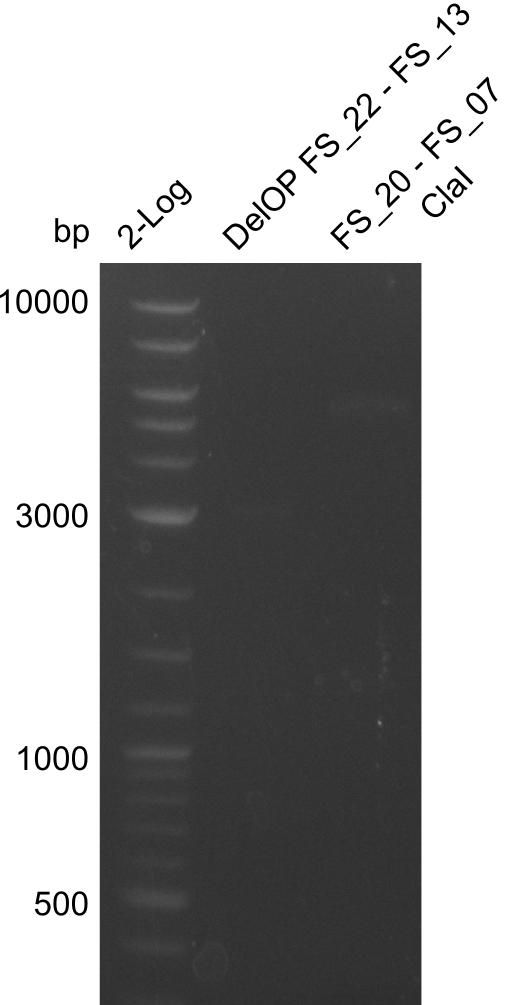
restriction digest of FS_20 to FS_07 (26.07.13) with ClaI and concentration measurement of DelOP (25.07.13); run at 100 V, 0.8 % gel (TAE)
Incubation at 37°C for 45 min
Results:
- restriction digest of Del FG did not lead to the expected results
- as no DNA was visible in the restriction digest, experiment will be repeated with a higher amount of DNA
Amplification from FS_06 to FS_07; 5.2 kb
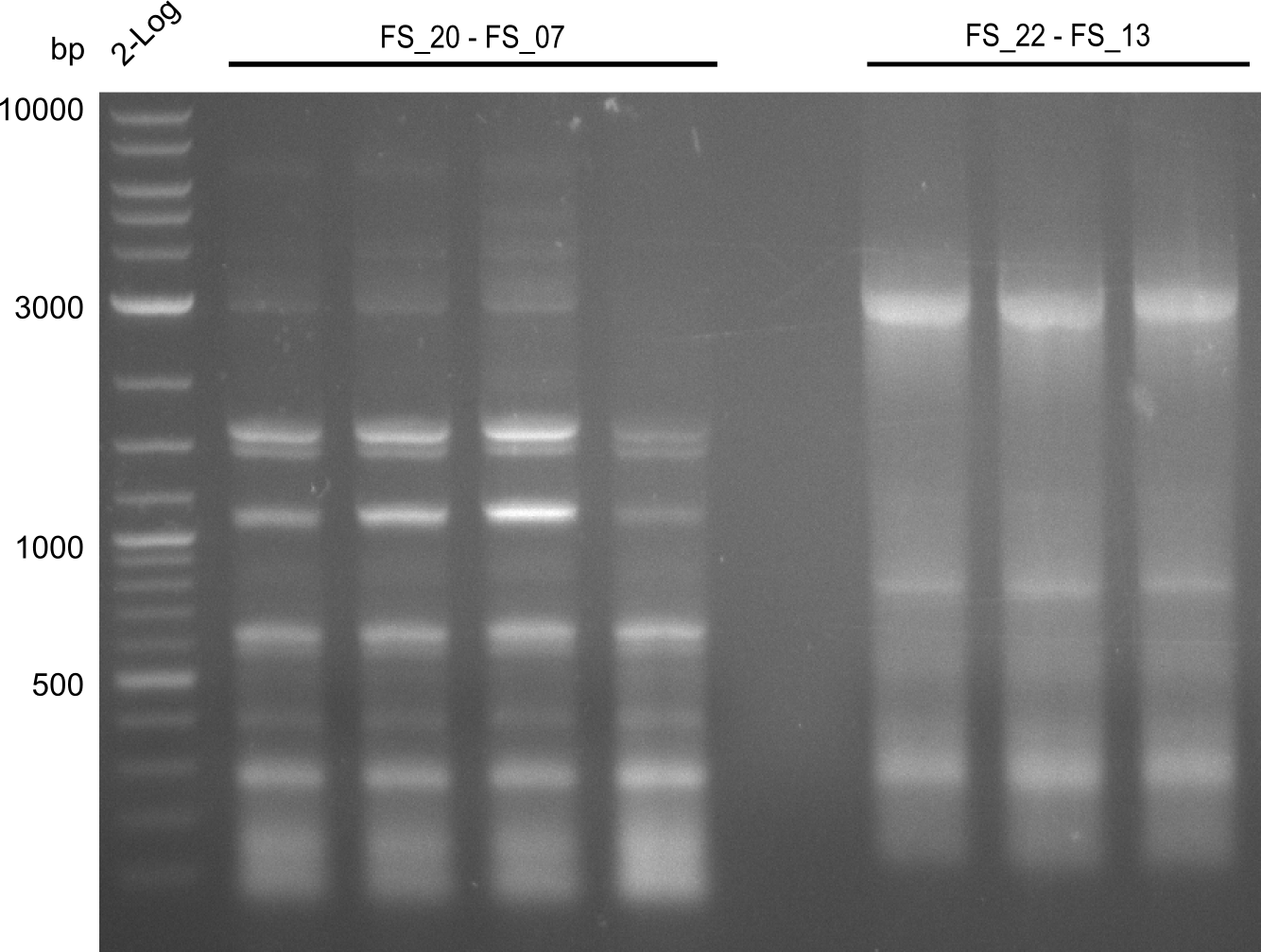
4 x Amplification of DelFG (FS20 to FS07; 26.07), 4 x Amplification of DelOP (FS_22 to FS13; 25.07); run at 100 V, 0.8 % gel (TAE)
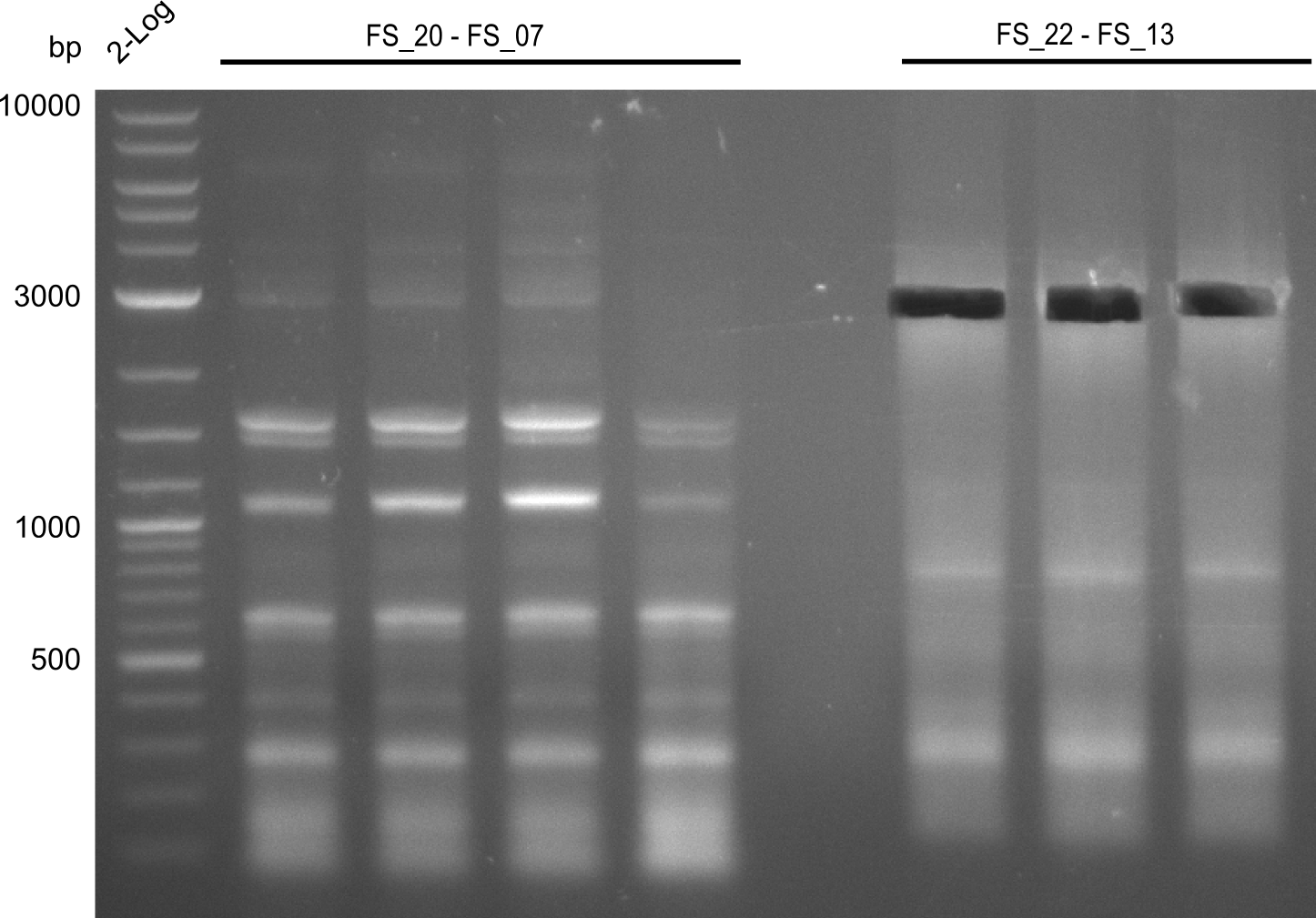
4 x Amplification of DelFG (FS20 to FS07; 26.07), 4 x Amplification of DelOP (FS_22 to FS13; 25.07) after cutting; run at 100 V, 0.8 % gel (TAE)
4 x 20µL
- Reaction I
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_20: (1/10) | 4
|
| FS_07: (1/10) | 4
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
- Conditions
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 72 ↓ 0.5 | 5
|
| 72 | 2:10
|
| 18 | 98 | 1
|
| 70 | 5
|
| 72 | 2:10
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelFG did not work, several bands occured, one of these had the size of the intended product but purity of the PCR was not sufficient for Gibson Assembly
- nethertheless bands were cut out and DNA purified using QIAquick Gel Extraction Kit for restriction digest
- Does anyone know, why we are constantly repeating this totally deficient PCR?
Amplification from FS_6/FS_20/FS_21 to FS_24 (PRIMER FS_24 MIXED UP!)
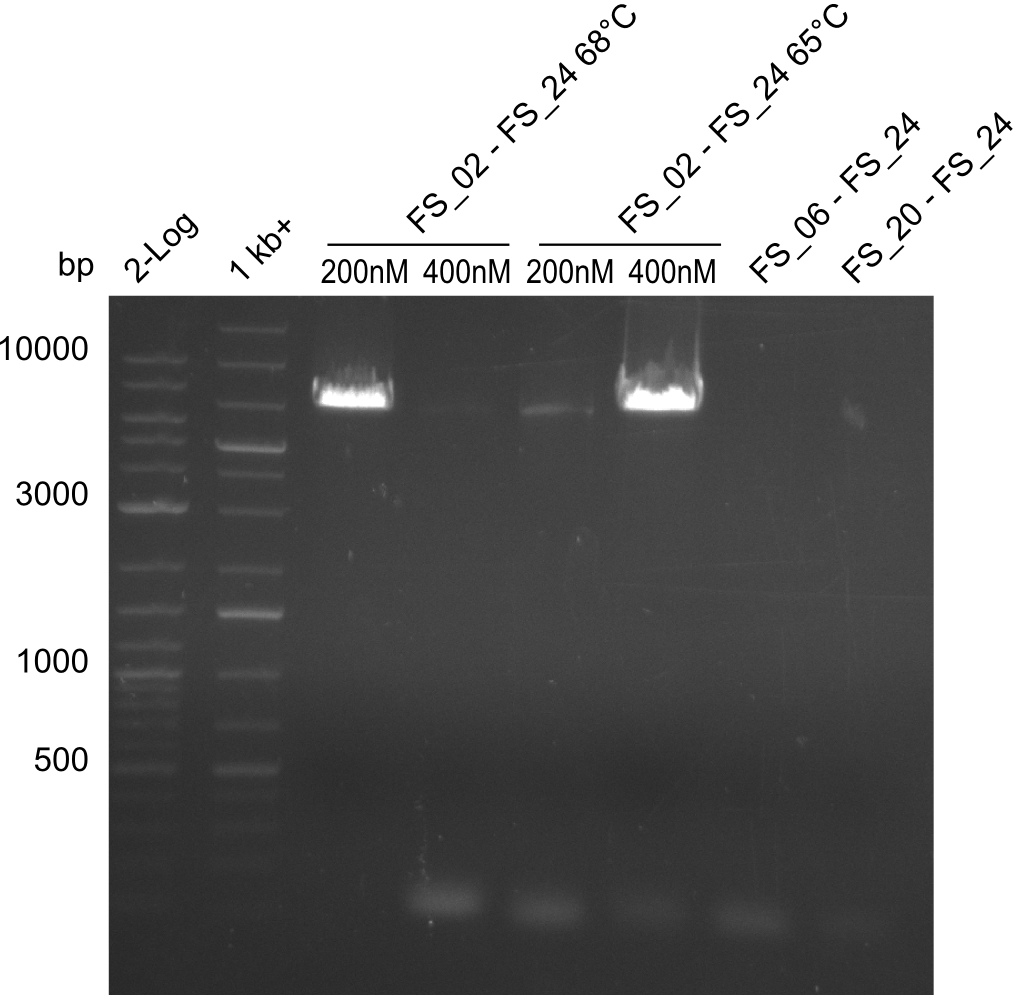
run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_06/FS_20/FS_21: (1/10) | 2
|
| FS_24: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 65 | 5
|
| 72 | 1:20
|
| 1 | 72 | 7 min
|
| 1 | 10 | inf
|
Results:
- no PCR product occured since the wrong primers were used
28-07-2013
Amplification from FS_21 to FS_24; (PRIMER 24 was MIXED UP!)
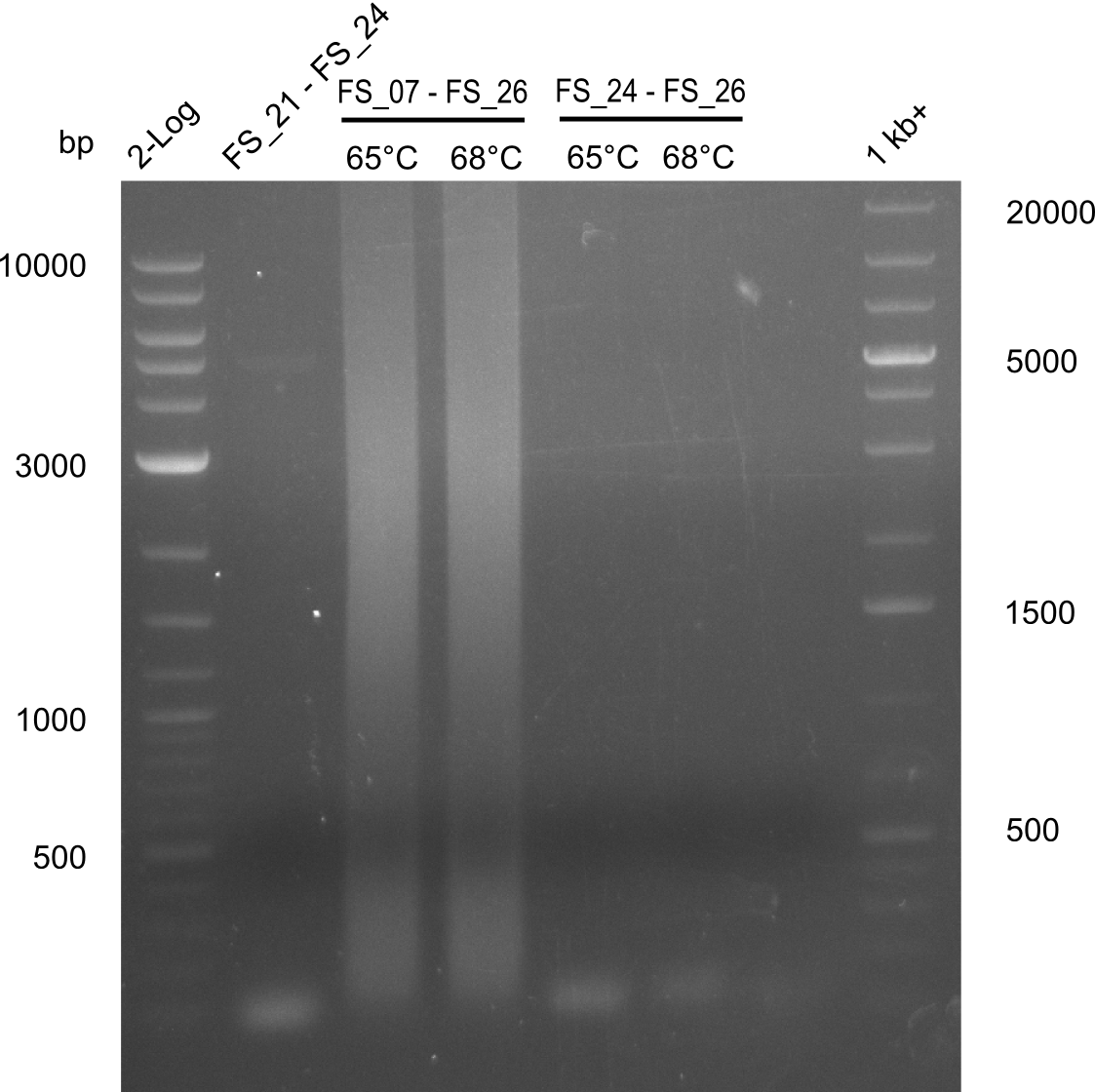
2log ladder / FS21-FS24 60const / FS07-FS26 65const / FS07-FS26 68const / FS24-FS26 65const / FS24-FS26 68const; run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_21: (1/10) | 2
|
| FS_24: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 58 | 5
|
| 72 | 2:30
|
| 1 | 72 | 8 min
|
| 1 | 12 | inf
|
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_21: (1/10) | 2
|
| FS_24: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 60 | 5
|
| 72 | 2:30
|
| 1 | 72 | 8 min
|
| 1 | 12 | inf
|
Results:
- no PCR product occured since the wrong primers were used
 "
"











