Team:UGent/Project
From 2013.igem.org
Contents |
Introduction: high gene expression in industrial biotechnology
The main goal of industrial biotechnology is to increase the yield of biochemical products using microorganisms as production hosts. This includes engineering large synthetic pathways and improving their expression. Overexpression of genes has mainly been achieved by using high or medium copy plasmids. However, studies have demonstrated that plasmid-bearing cells lose their productivity fairly quickly as a result of genetic instability.
In industrial biotechnology, a common technique to express new synthetic products and pathways is the use of plasmids as vectors. Plasmids are easy to insert into cells and replicate independently from the genome, allowing strong gene expression. Overexpression is easily achieved by using plasmids with a medium or high copy number, different promoter systems, ribosome binding sites (RBS), etc. Thanks to plasmids, the industrial biotechnology has grown substantially over the past years. However, the use of plasmids entails some important disadvantages.
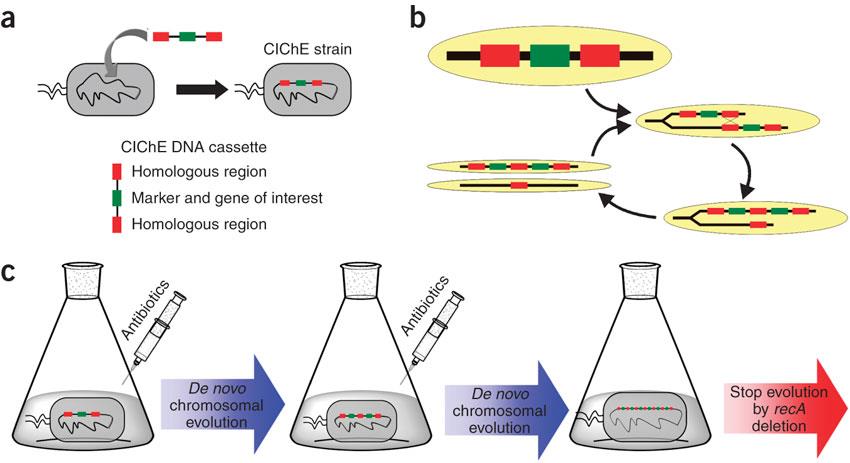
CloseIn 2009, Tyo et al. developed a technique for the stable, high copy expression of a gene of interest in E. coli without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics2. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation. CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker chloramphenicol acetyl transferase (cat) flanked by homologous regions, is delivered to and subsequently integrated into the E. coli genome. The construct can be amplified in the genome through tandem gene duplication by recA homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (Figure).
This process is called chromosomal evolution. When the desired gene copy number is reached, recA is deleted, thereby fixing the copy number.CloseOur model
Toxin-Antitoxin systems
The need for antibiotics may also be eliminated by using toxin-antitoxin systems (TA). These systems are widely distributed among bacteria and archaea. The toxins produced by these systems can slow down or stop cell growth by interfering with certain molecules that are essential in cellular processes like DNA replication, cell wall synthesis, ATP synthesis etc.. Under normal conditions, the toxin is inhibited by the antitoxin, which is encoded in the same operon as the toxin. Research revealed different types of TA systems, which will be discussed below.
Read more about the different types of TA systems
Type I
The first category of TA systems is characterised by an antisense mechanism that regulates the expression of the toxin gene31. This antisense RNA is transcribed from the same toxin region, but in reversed orientation, and encodes the antitoxin. This antitoxin anneals to the toxin mRNA and forms double-stranded RNA across the ribosome binding site32. This induces the degradation of the toxin mRNA or the blocking of the ribosome binding site. As a result, the amount of toxin in the cell is reduced.
Type II
Antitoxins in type II TA systems are proteins and inhibit the corresponding toxin by forming a stable TA protein complex. These systems are encoded by an operon that contains the two genes encoding the toxin and antitoxin. The antitoxin has to be continuously produced, as it is less stable than the toxin. Because of this feature, the cell has a feedback mechanism to prevent growth arrest. The TA complex functions as a repressor that binds the promoter and thereby inhibits transcription. Thus, when the antitoxin is degraded and the concentration of the TA complex decreases, the TA operon is derepressed and produces more toxin and antitoxin. Finally, the concentration of the antitoxin is reestablished. The rank of the two genes differs, which can contribute to different toxin-antitoxin ratios.
Type III
In the last category of TA systems, an RNA antitoxin directly inhibits the toxin. These RNA antitoxins are pseudoknots, which contain internal stemloops. The toxin and its antidote are once more encoded by the same operon. A transcriptional terminator between the two genes, regulates the ratio of toxin and antitoxin in the cell34. Figure 17: TA systems type III mechanism. The antitoxin RNA directly inhibits the toxin protein. A transcriptional terminator regulates the relative amounts of toxin and antitoxin in the cell.
CloseResults

Tweets van @iGEM_UGent
 "
"