Team:Paris Saclay/Notebook/August/12
From 2013.igem.org
Contents |
Notebook : August 12
Lab work
A - Aerobic/Anaerobic regulation system
Obtaining Pfnr_RBS-LacZ-Term in PSB1C3
1 - Digestion of Bba_K1155000, Bba_K1155007 and Bba_K1155003
Anaïs, Nadia, XiaoJing
Protocol : digestion
Used quantities :
- Bba_K1155000 :
- Buffer : 5µL
- H2O : 38µL
- Pfnr : 5µL
- SpeI : 1µL
- PstI : 1µL
- Bba_K1155007 :
- Buffer : 5µL
- H2O : 23µL
- RBS-LacZ-Term : 20µL
- XBal : 1µL
- PstI : 1µL
- Bba_K1155003 :
- Buffer : 5µL
- H2O : 33µL
- RBS-AmilCP-Term : 10µL
- XBal : 1µL
- PstI : 1µL
| style="width:350px;border:1px solid black;" | 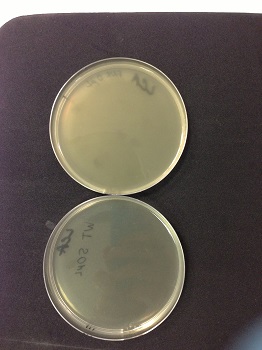 | style="width:350px;border:1px solid black;vertical-align:top;" |
Picture: lysed cells comparison.
| style="width:350px;border:1px solid black;vertical-align:top;" |
Picture: lysed cells comparison.
- 0µl phage(control): the petri dish is cloudy, bacteria are not lysed.
- 50µl phage: the petri dish is clear, bacteria are lysed by phages.
- We used a wild type strain to keep a stock and a Δ fnr E. coli strain to obtain phages that might have encapsidated a Δfnr::Km fragment.
|}
10µl,50µl and 100µl petri dishes are clear so phages are multiplied.
We let the antibiotic over night to select the right strain.
2 - ObtaµLining RBS_lacZ_ter.
Abdou
Clone 10,14 and 15 plamid extraction using nucleospin kit.
Protocol : hight copy plamid extraction
Results: concentration measured by nanodrop
- clone 10: C=38ng/µl 260/280= 1.78
- clone 14: C=48.5ng/µl 260/280=1.90
- clone 15: C=52 ng/µl 260/280=1.78
We still have to sequence plasmids in order to verify our results.
A - Aerobic/Anaerobic regulation system / B - PCB sensing system
1 - Gibson assembly.
August 1st PCR purification to be sure about the experiment. BphR2 part1, BphR2 part2 and RBS_BphR2_part1, FNR part1, FNR part2 and RBS_FNR_part1.
Protocol : PCR_clean_up
Results:
| IMAGE |
|
we have no fragment so we must do again these PCRs
 "
"