From 2013.igem.org
Aug 22
Liquid culture
Nakamoto
| Sample | Medium
|
| 8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT (2) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Pcon-RBS-GFP-DT control (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Pcon-RBS-GFP-DT control (2) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 RBS-lysis1 (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 RBS-lysis1 (2) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 RBS control (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 RBS control (2) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Ptet(pm)-RBS-tetR-DT (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Ptet(pm)-RBS-tetR-DT (2) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Ptet(pm) control (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Ptet(pm) control (2) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 Plux-RBS-GFP-DT (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 pSB1C3(BBa_J04450) (1) | 8/21 Plusgrow medium (+Amp)
|
| 8/21 pSB1C3(BBa_J04450) (2) | 8/21 Plusgrow medium (+Amp)
|
incubate 37°C 10hour
Colony PCR
Kojima
| Sample | base pair
|
| 8/21 RBS-lysis1(1) | 400
|
| 8/21 RBS-lysis1(2) | 400
|
| 8/21 RBS control(1) | --
|
| 8/21 RBS control(2) | --
|
| negative control | --
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 94°C | 55°C | 68°C | --
|
| 5min | 30s | 30s | 30s | 30cycles
|
| Sample | base pair
|
| 8/21 Pcon-GFP-DT-Pcon-RBS-luxR-DT (1) | 2143
|
| 8/21 Pcon-GFP-DT-Pcon-RBS-luxR-DT (2) | 2143
|
| 8/21 Pcon-RBS-luxR control(1) | --
|
| 8/21 Pcon-RBS-luxR control(2) | --
|
| negative control | --
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 94°C | 55°C | 68°C | --
|
| 5min | 30s | 30s | 2min | 30cycles
|
| Sample | base pair
|
| 8/21 Ptet-RBS-tetR-DT (1) | 1216
|
| 8/21 Ptet-RBS-tetR-DT (2) | 1216
|
| 8/21 Ptet control (1) | --
|
| 8/21 Ptet control (2) | --
|
| 8/21 Plux-RBS-GFP-DT (1) | 1227
|
| 8/21 pSB1C3(BBa_J04450) (1) | 1353
|
| 8/21 pSB1C3(BBa_J04450) (2) | 1353
|
| negative control | --
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 94°C | 55°C | 68°C | --
|
| 5min | 30s | 30s | 1min | 30cycles
|
Electrophoresis
No name
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | pT181antisense | EcoRI | SpeI
|
| 2 | pT181antisense | EcoRI | --
|
| 3 | pT181antisense | -- | SpeI
|
| 4 | pT181antisense | -- | --
|
| 5 | pT181antisense | XbaI | PstI
|
| 6 | pT181antisense | XbaI | --
|
| 7 | pT181antisense | -- | PstI
|
| 8 | pT181antisense | -- | --
|
| 9 | 100bp ladder | -- | --
|
| 10 | Fusion1 attenuator | EcoRI | SpeI
|
| 11 | Fusion1 attenuator | EcoRI | --
|
| 12 | Fusion1 attenuator | -- | SpeI
|
| 13 | Fusion1 attenuator | -- | --
|
| 14 | Fusion1 attenuator | XbaI | PstI
|
| 15 | Fusion1 attenuator | XbaI | --
|
| 16 | fusion1 attenuator | -- | PstI
|
| 17 | fusion1 attenuator | -- | --
|
File:Igku xxxxxx.xxx
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | Fusion3 3m2 attenuator | EcoRI | SpeI
|
| 2 | Fusion3 3m2 attenuator | EcoRI | --
|
| 3 | Fusion3 3m2 attenuator | -- | SpeI
|
| 4 | Fusion3 3m2 attenuator | -- | --
|
| 5 | Fusion3 3m2 attenuator | XbaI | PstI
|
| 6 | Fusion3 3m2 attenuator | XbaI | --
|
| 7 | Fusion3 3m2 attenuator | -- | PstI
|
| 8 | Fusion3 3m2 attenuator | -- | --
|
| 9 | 100bp ladder | -- | --
|
| 10 | aptamer 12-1M | EcoRI | SpeI
|
| 11 | aptamer 12-1M | EcoRI | --
|
| 12 | aptamer 12-1M | -- | SpeI
|
| 13 | aptamer 12-1M | -- | --
|
| 14 | aptamer 12-P | EcoRI | SpeI
|
| 15 | aptamer 12-P | EcoRI | --
|
| 16 | aptamer 12-1P | -- | SpeI
|
| 17 | aptamer 12-1P | -- | --
|
File:Igku xxxxxx.xxx
Restriction Enzyme Digestion
Tatsui, Kojima, Honda and Ashida
| | Fusion1 antisense (1) (300 µg/mL) | EcoRI | SpeI | buffer H | MilliQ | total
|
| 2 cuts | 3.3 | 1 | 1 | 3 | 21.7 | 30
|
| 1 cut | 0.7 | 0.2 | 0 | 1 | 8.1 | 10
|
| 1 cut | 0.7 | 0 | 0.2 | 1 | 8.1 | 10
|
| NC | 0.7 | 0 | 0 | 1 | 8.3 | 10
|
| | Fusion1 antisense (1) (300 µg/mL) | XbaI | PstI | buffer M | BSA | MilliQ | total
|
| 2 cuts | 3.3 | 1 | 1 | 3 | 3 | 18.7 | 30
|
| 1 cut | 0.7 | 0.2 | 0 | 1 | 1 | 7.1 | 10
|
| 1 cut | 0.7 | 0 | 0.2 | 1 | 1 | 7.1 | 10
|
| NC | 0.7 | 0 | 0 | 1 | 1 | 7.3 | 10
|
| | Fusion6 antisense (1) (336 µg/mL) | EcoRI | SpeI | buffer H | MilliQ | total
|
| 2 cuts | 3 | 1 | 1 | 3 | 22 | 30
|
| 1 cut | 0.6 | 0.2 | 0 | 1 | 8.2 | 10
|
| 1 cut | 0.6 | 0 | 0.2 | 1 | 8.2 | 10
|
| NC | 0.6 | 0 | 0 | 1 | 8.4 | 10
|
| | Fusion6 antisense (1) (336 µg/mL) | XbaI | PstI | buffer M | BSA | MilliQ | total
|
| 2 cuts | 3 | 1 | 1 | 3 | 3 | 19 | 30
|
| 1 cut | 0.6 | 0.2 | 0 | 1 | 1 | 7.2 | 10
|
| 1 cut | 0.6 | 0 | 0.2 | 1 | 1 | 7.2 | 10
|
| NC | 0.6 | 0 | 0 | 1 | 1 | 7.4 | 10
|
| | pT181 antisense (1) (178 µg/mL) | EcoRI | SpeI | buffer H | MilliQ | total
|
| 2 cuts | 5.6 | 1 | 1 | 3 | 19.4 | 30
|
| 1 cut | 1.1 | 0.2 | 0 | 1 | 7.7 | 10
|
| 1 cut | 1.1 | 0 | 0.2 | 1 | 7.7 | 10
|
| NC | 1.1 | 0 | 0 | 1 | 7.9 | 10
|
| | pT181 antisense (1) (178 µg/mL) | XbaI | PstI | buffer M | BSA | MilliQ | total
|
| 2 cuts | 5.6 | 1 | 1 | 3 | 3 | 16.4 | 30
|
| 1 cut | 1.1 | 0.2 | 0 | 1 | 1 | 6.7 | 10
|
| 1 cut | 1.1 | 0 | 0.2 | 1 | 1 | 6.7 | 10
|
| NC | 1.1 | 0 | 0 | 1 | 1 | 6.9 | 10
|
| | pT181 attenuator (1) | EcoRI | SpeI | buffer H | MilliQ | total
|
| 2 cuts | 3 | 1 | 1 | 3 | 22 | 30
|
| 1 cut | 0.6 | 0.2 | 0 | 1 | 8.2 | 10
|
| 1 cut | 0.6 | 0 | 0.2 | 1 | 8.2 | 10
|
| NC | 0.6 | 0 | 0 | 1 | 8.4 | 10
|
| | pT181 attenuator (1) | XbaI | PstI | buffer M | BSA | MilliQ | total
|
| 2 cuts | 3 | 1 | 1 | 3 | 3 | 19 | 30
|
| 1 cut | 0.6 | 0.2 | 0 | 1 | 1 | 7.2 | 10
|
| 1 cut | 0.6 | 0 | 0.2 | 1 | 1 | 7.2 | 10
|
| NC | 0.6 | 0 | 0 | 1 | 1 | 7.4 | 10
|
| | Fusion1 attenuator (1) | EcoRI | SpeI | buffer H | MilliQ | total
|
| 2 cuts | 3.3 | 1 | 1 | 3 | 21.7 | 30
|
| 1 cut | 0.7 | 0.2 | 0 | 1 | 8.1 | 10
|
| 1 cut | 0.7 | 0 | 0.2 | 1 | 8.1 | 10
|
| NC | 0.7 | 0 | 0 | 1 | 8.3 | 10
|
| | Fusion1 attenuator (1) | XbaI | PstI | buffer M | BSA | MilliQ | total
|
| 2 cuts | 3.3 | 1 | 1 | 3 | 3 | 18.7 | 30
|
| 1 cut | 0.7 | 0.2 | 0 | 1 | 1 | 7.1 | 10
|
| 1 cut | 0.7 | 0 | 0.2 | 1 | 1 | 7.1 | 10
|
| NC | 0.7 | 0 | 0 | 1 | 1 | 7.3 | 10
|
| | Fusion3m2 attenuator (1) | EcoRI | SpeI | buffer H | MilliQ | total
|
| 2 cuts | 3 | 1 | 1 | 3 | 22 | 30
|
| 1 cut | 0.6 | 0.2 | 0 | 1 | 8.2 | 10
|
| 1 cut | 0.6 | 0 | 0.2 | 1 | 8.2 | 10
|
| NC | 0.6 | 0 | 0 | 1 | 8.4 | 10
|
| | Fusion3m2 attenuator (1) | XbaI | PstI | buffer M | BSA | MilliQ | total
|
| 2 cuts | 3 | 1 | 1 | 3 | 3 | 19 | 30
|
| 1 cut | 0.6 | 0.2 | 0 | 1 | 1 | 7.2 | 10
|
| 1 cut | 0.6 | 0 | 0.2 | 1 | 1 | 7.2 | 10
|
| NC | 0.6 | 0 | 0 | 1 | 1 | 7.4 | 10
|
| | Spinach (2) | EcoRI | SpeI | buffer B | BSA | MilliQ | total
|
| 2 cuts | 2.3 | 0.5 | 0.5 | 3 | 0.3 | 23.4 | 30
|
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10
|
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10
|
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10
|
| | tetR aptamer 12_P (2) | EcoRI | SpeI | buffer B | BSA | MilliQ | total
|
| 2 cuts | 2.7 | 0.5 | 0.5 | 3 | 0.3 | 23 | 30
|
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10
|
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10
|
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10
|
| | tetR aptamer 12_1R (2) | EcoRI | SpeI | buffer B | BSA | MilliQ | total
|
| 2 cuts | 2.6 | 0.5 | 0.5 | 3 | 0.3 | 23.1 | 30
|
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10
|
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10
|
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10
|
| | tetR aptamer 12_1M (1) | EcoRI | SpeI | buffer B | BSA | MilliQ | total
|
| 2 cuts | 2.7 | 0.5 | 0.5 | 3 | 0.3 | 23 | 30
|
| 1 cut | 0.5 | 0.1 | 0 | 1 | 0.1 | 8.3 | 10
|
| 1 cut | 0.5 | 0 | 0.1 | 1 | 0.1 | 8.3 | 10
|
| NC | 0.5 | 0 | 0 | 1 | 0.1 | 8.4 | 10
|
| | J23100 | SpeI | PstI | buffer B | BSA | MilliQ | total
|
| 2 cuts | 7 | 0.5 | 0.5 | 3 | 0.3 | 18.7 | 30
|
| 1 cut | 1.4 | 0.1 | 0 | 1 | 0.1 | 7.4 | 10
|
| 1 cut | 1.4 | 0 | 0.1 | 1 | 0.1 | 7.4 | 10
|
| NC | 1.4 | 0 | 0 | 1 | 0.1 | 7.5 | 10
|
| | Plac (2) | SpeI | PstI | buffer B | BSA | MilliQ | total
|
| 2 cuts | 11.2 | 0.5 | 0.5 | 3 | 0.3 | 14.5 | 30
|
| 1 cut | 2.2 | 0.1 | 0 | 1 | 0.1 | 6.6 | 10
|
| 1 cut | 2.2 | 0 | 0.1 | 1 | 0.1 | 6.6 | 10
|
| NC | 2.2 | 0 | 0 | 1 | 0.1 | 6.7 | 10
|
Miniprep
Ashida
| DNA | concentration[µg/mL] | 260/280 | 260/230
|
| 8/22 RBS-lysis (1) | 128 | 1.77 | 2.29
|
| 8/22 RBS-lysis (2) | 96 | 1.80 | 2.07
|
| 8/22 pSB1C3 (1) | 108 | 1.55 | 1.45
|
| 8/22 pSB1C3 (2) | 130 | 1.69 | 1.76
|
Electrophoresis
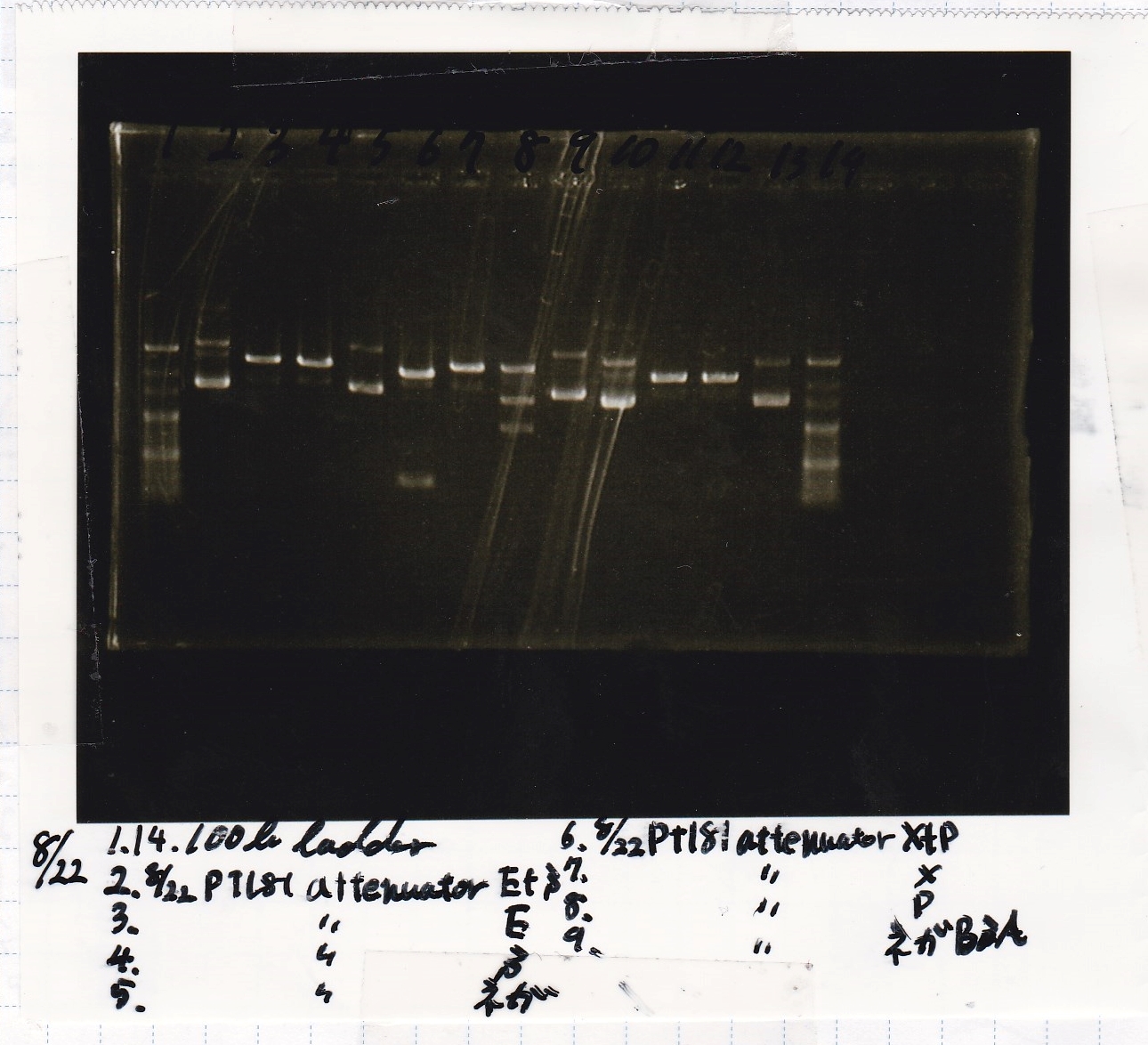
No name
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | 100bp | -- | --
|
| 2 | 8/22 pT181 attenuator (1) | EcoRI | SpeI
|
| 3 | 8/22 pT181 attenuator (1) | EcoRI | --
|
| 4 | 8/22 pT181 attenuator (1) | -- | SpeI
|
| 5 | 8/22 pT181 attenuator (1) | -- | --
|
| 6 | 8/22 pT181 attenuator (1) | XbaI | PstI
|
| 7 | 8/22 pT181 attenuator (1) | XbaI | --
|
| 8 | 8/22 pT181 attenuator (1) | -- | PstI
|
| 9 | 8/22 pT181 attenuator (1) | -- | --
|
| 10 | 8/22 Fusion6 antisense (1) | EcoRI | SpeI
|
| 11 | 8/22 Fusion6 antisense (1) | EcoRI | --
|
| 12 | 8/22 Fusion6 antisense (1) | -- | SpeI
|
| 13 | 8/22 Fusion6 antisense (1) | -- | --
|
| 14 | 100bp | -- | --
|
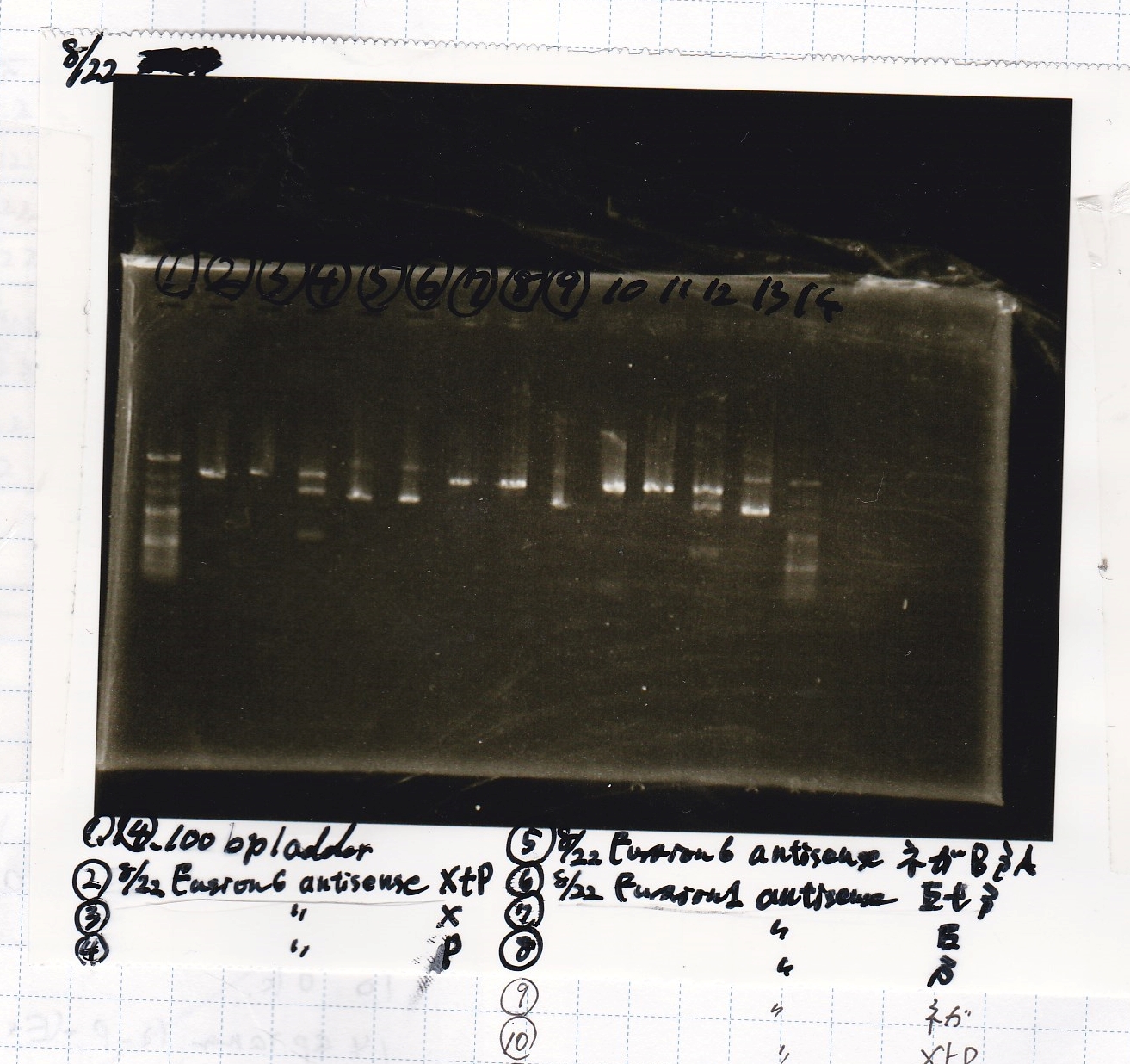
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | 100bp | -- | --
|
| 2 | 8/22 Fusion6 antisense (1) | XbaI | PstI
|
| 3 | 8/22 Fusion6 antisense (1) | XbaI | --
|
| 4 | 8/22 Fusion6 antisense (1) | -- | PstI
|
| 5 | 8/22 Fusion6 antisense (1) | -- | --
|
| 6 | 8/22 Fusion1 antisense (1) | EcoRI | SpeI
|
| 7 | 8/22 Fusion1 antisense (1) | EcoRI | --
|
| 8 | 8/22 Fusion1 antisense (1) | -- | SpeI
|
| 9 | 8/22 Fusion1 antisense (1) | -- | --
|
| 10 | 8/22 Fusion1 antisense (1) | XbaI | PstI
|
| 11 | 8/22 Fusion1 antisense (1) | XbaI | --
|
| 12 | 8/22 Fusion1 antisense (1) | -- | PstI
|
| 13 | 8/22 Fusion1 antisense (1) | -- | --
|
| 14 | 100bp | -- | --
|
 "
"

