Template:Kyoto/Notebook/Sep 3
From 2013.igem.org
Contents |
Sep 3
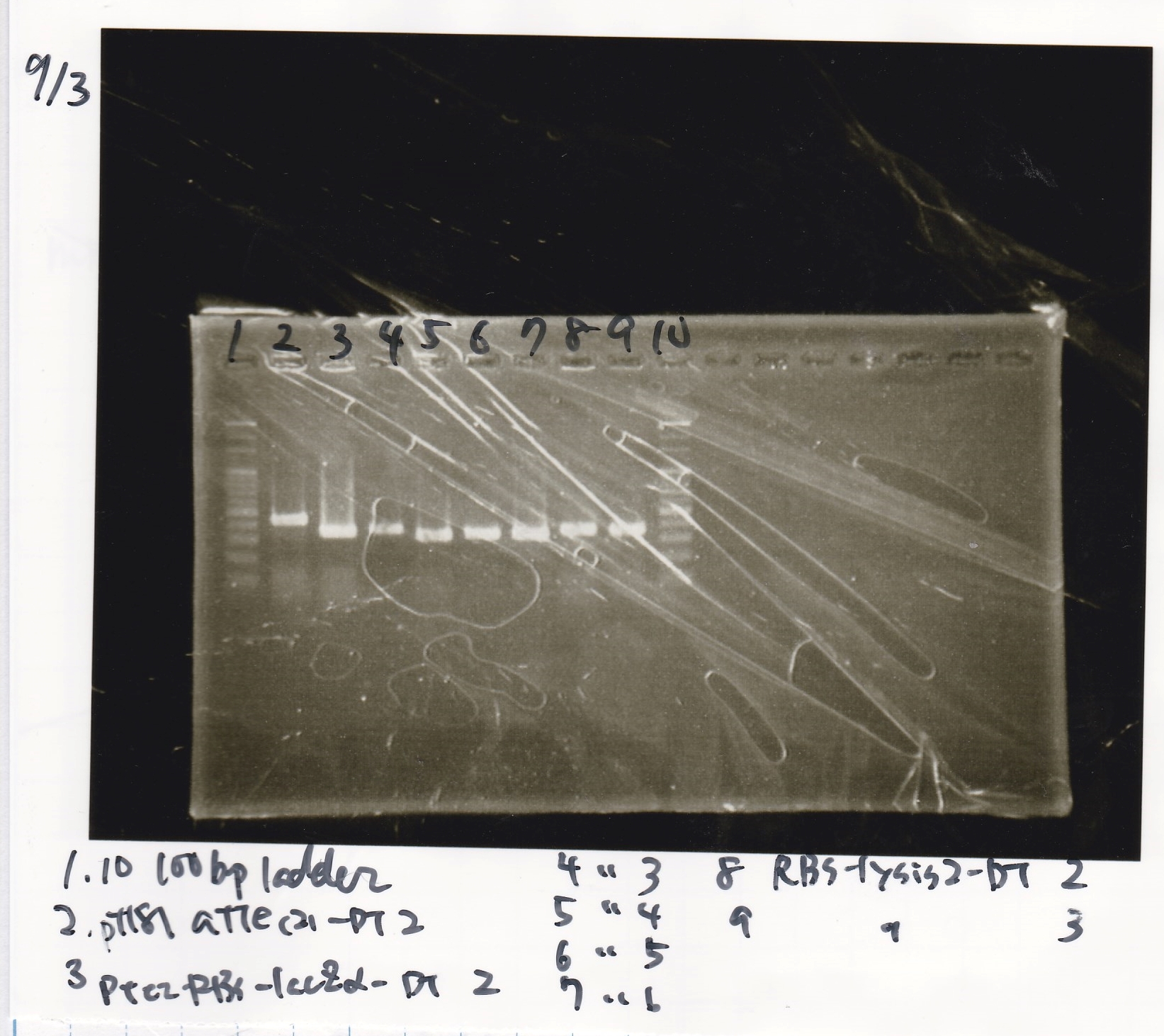
Electrophoresis
| Lane | Sample |
|---|---|
| 1 | 100bp ladder |
| 2 | pT181 attenuater(2)-DT2(738) |
| 3 | Ptet-RBS-lacZα-DT-2(756) |
| 4 | Ptet-RBS-lacZα-DT-3 |
| 5 | Ptet-RBS-lacZα-DT-4 |
| 6 | Ptet-RBS-lacZα-DT-5 |
| 7 | Ptet-RBS-lacZα-DT-6 |
| 8 | RBS-lysis2-DT-2(985) |
| 9 | RBS-lysis2-DT-3 |
| 10 | 100bp ladder |
Miniprep
| DNA | concentration[µg/mL] | 260/280 | 260/230 |
|---|---|---|---|
| 9/2 14:00 pT181 attenuator | 418.4 | 1.82 | 1.80 |
| 9/2 14:00 pT181 antisense | 362.2 | 1.92 | 1.96 |
| 9/2 14:00 Spinach | 438.3 | 1.86 | 1.99 |
| 9/2 14:00 tetR-apt 12_1R | 567.5 | 1.90 | 1.99 |
| 9/2 21:00 pT181 attenuater | 320.9 | 1.90 | 2.15 |
| 9/2 21:00 Spinach | 457.0 | 1.88 | 2.24 |
Liquid Culture
| Sample | medium |
|---|---|
| Spinach-DT(9/2 master plate) | plusgrow(CP)4ml |
Restriction Enzyme Digestion
| DNA | EcoR1 | Spe1 | Xba1 | Pst1 | 10xBuffer B(EcoR1&Spe1) | 10xBuffer D(Xba1&Pst1) | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| pT181attenuater 14:00 418.4ng/µL | 4.8µL | 1.0µL | 1.0µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 19.9µL | 30µL |
| pT181attenuater 14:00 418.4ng/µL | 0.2µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.7µL | 10µL |
| pT181attenuater 14:00 418.4ng/µL | 4.8µL | 0µL | 0µL | 1.0µL | 1.0µL | 0µL | 3µL | 0.3µL | 19.9µL | 30µL |
| pT181attenuater 14:00 418.4ng/µL | 0.2µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.7µL | 10µL |
| DNA | EcoR1 | Spe1 | Xba1 | Pst1 | 10xBuffer B(EcoR1&Spe1) | 10xBuffer D(Xba1&Pst1) | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| pT181antisense 14:00 362.2ng/µL | 5.5µL | 1.0µL | 1.0µL | 0µL | 0µL | 3µL | 0µL | 0.3µL | 19.2µL | 30µL |
| pT181attenuater 14:00 418.4ng/µL | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 0.1µL | 8.6µL | 10µL |
| pT181attenuater 14:00 418.4ng/µL | 5.5µL | 0µL | 0µL | 1.0µL | 1.0µL | 0µL | 3µL | 0.3µL | 19.2µL | 30µL |
| pT181attenuater 14:00 418.4ng/µL | 0.3µL | 0µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0.1µL | 8.6µL | 10µL |
| DNA | EcoR1 | Spe1 | 10xBuffer B | 100xBSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| apt 12_1R 14:00 507.5ng/µL | 3.9µL | 1.0µL | 1.0µL | 3µL | 0.3µL | 20.8µL | 30µL |
| apt 12_1R 14:00 507.5ng/µL | 0.2µL | 0µL | 0µL | 1µL | 0.1µL | 8.7µL | }
PCRMutatation PCRColony PCRRestriction Enzyme DigestionGel ExtractionLiquid Culture |
 "
"