Team:UC-Santa Cruz/Notebook
From 2013.igem.org
2013 UCSC IGEM TEAM Polar Tags Notebook.
Index-
Topic Page #
Groups Goals
The goal of the Polar Tag Group is to
General PCR Protocol
General Agarose Gel Protocol
General Chemicompetent Cell Transformation Protocol
LB Media Recipe Making LB+Kanamycin and LB+Gentamicin Agar Plates: To make a 500 ml batch: - 475 ml of MilliQ water - 5 g of Tryptone - 5 g of NaCl - 2.5 g of yeast Extract - 7.5 g of Agar We made x2 500 ml batches. Combine the reagents and shake until the solutes have dissolved. Adjust the ph to 7.0. Sterilize by autoclaving for 20 minutes on liquid cycle. Take out of the autoclave and let the solution cool. Place in a water bath to prevent solid formation. Add 1.25 ml of gentamicin antibiotic to one of the 500 ml batches and 500 ul of kanamycin to the other batch. Pour into petri dishes about half way. Let agar solidify. Place in the refrigerator.
Colony Pick Protocol
Electroporation Protocol
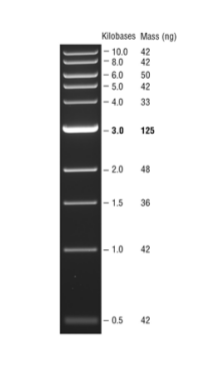
DNA MW marker reference
Reference Sequences
References
Lab Notebook 8/6/13
Lab Notebook 8/7/13
Lab Notebook 8/8/13
Lab Notebook 8/9/13
Lab Notebook 8/13/13
Lab Notebook 8/15/13
Lab Notebook 8/16/13
Lab Notebook 8/19/13
Lab Notebook 8/20/13
Lab Notebook 8/21/13
Lab Notebook 8/22/13
Lab Notebook 8/25/13
Lab Notebook 8/26/13
Lab Notebook 8/27/13
Lab Notebook 8/28/13
Lab Notebook 8/29/13
Lab Notebook 8/30/13
Lab Notebook 8/31/13
Lab Notebook 9/1/13
Lab Notebook 9/2/13
Lab Notebook 9/3/13
Lab Notebook 9/4-5/13
Lab Notebook 9/6/13
Lab Notebook 9/7/13
Making Top10 Chemicompetent Cells: 1) Grow Top10 SOB overnight between the temperatures 20°C and 33°C. 2) Measure the OD and bring the OD back to 0.1. 3) Add 2.5 ml of Top10 SOB to SOB. 4) Incubate at 31°C for one hour and 20 minutes or until the OD is 0.6. 5) Create 5 ml of 1X Washing buffer: 2.5 ml of Dilution buffer and 2.5 ml of Wash buffer (2X) 6) Create 5 ml of 1X Competent buffer: 2.5 ml Dilution buffer and 2.5 ml of Competent buffer (2X) 7) Incubate the culture on ice for ten minutes and then pellet cells at 2,500 xg for ten minutes at 0-4°C. 8) Remove supernatant. Gently resuspend cells in 5 ml of ice cold 1X Wash buffer. Re-pellet as in step 7. 9) Remove supernatant completely. Gently resuspend cells in 5 ml of ice cold 1X Competent buffer. 10) Aliquot 100-200 ul. Flash freeze. 11) Store in -80°C freezer.
Making LB+Kanamycin and LB+Gentamicin Agar Plates: To make a 500 ml batch: - 475 ml of MilliQ water - 5 g of Tryptone - 5 g of NaCl - 2.5 g of yeast Extract - 7.5 g of Agar We made x2 500 ml batches. Combine the reagents and shake until the solutes have dissolved. Adjust the ph to 7.0. Sterilize by autoclaving for 20 minutes on liquid cycle. Take out of the autoclave and let the solution cool. Place in a water bath to prevent solid formation. Add 1.25 ml of gentamicin antibiotic to one of the 500 ml batches and 500 ul of kanamycin to the other batch. Pour into petri dishes about half way. Let agar solidify. Place in the refrigerator.
Lab Notebook 9/8/13
Lab Notebook 9/10/13
Lab Notebook 9/11/13
Lab Notebook 9/12/13
Lab Notebook 9/13/13
Lab Notebook 9/14/13
Lab Notebook 9/15/13
Lab Notebook 9/16/13
Lab Notebook 9/17/13
Lab Notebook 9/18/13
Lab Notebook 9/19/13
Lab Notebook 9/20/13
 "
"