Team:Heidelberg/Templates/Delftibactin week20
From 2013.igem.org
Contents |
09-09 - 15-09-13
Optimizing Growth Conditions of D.acidovorans and Evaluation of Endogenous Background Gold Precipitation
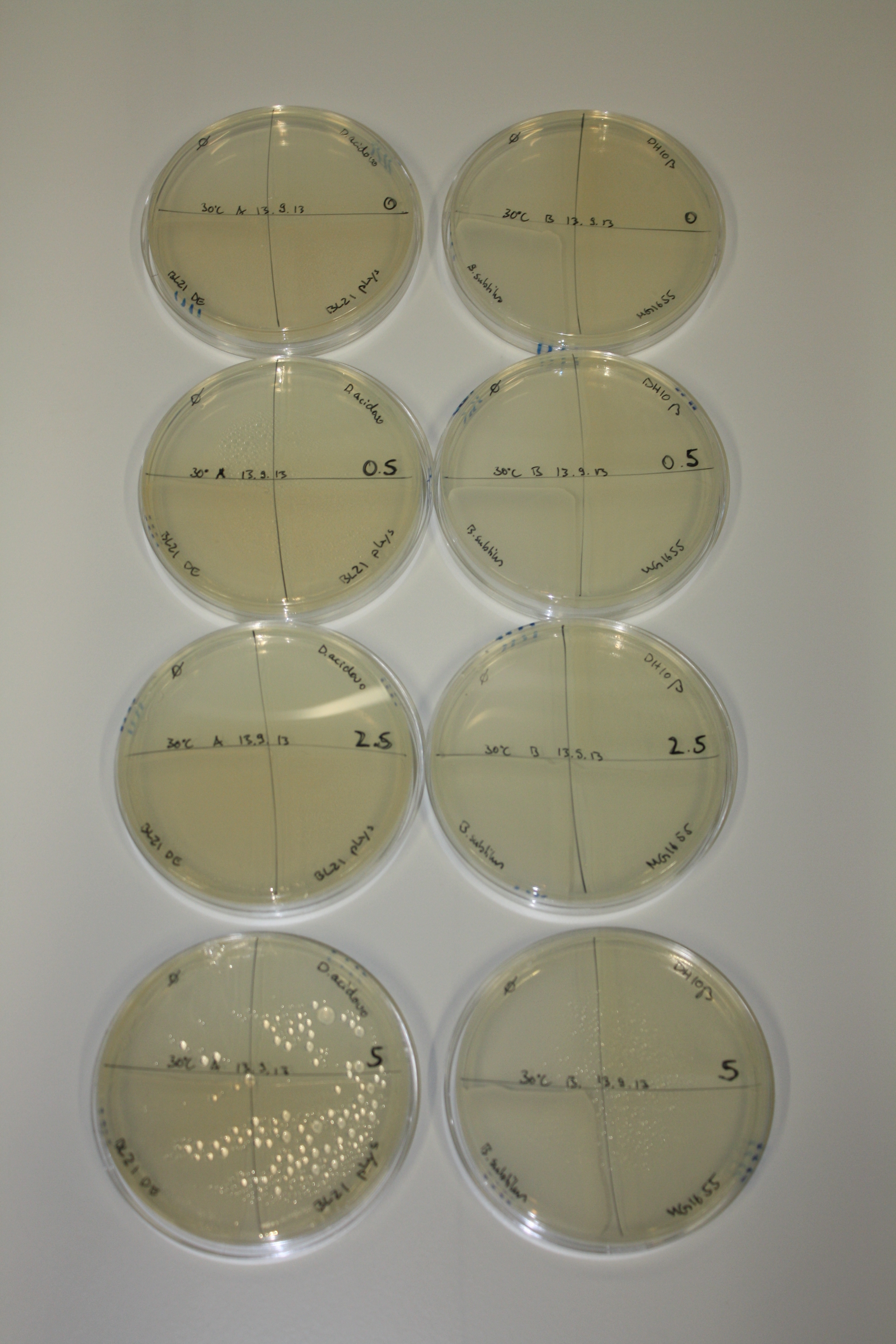
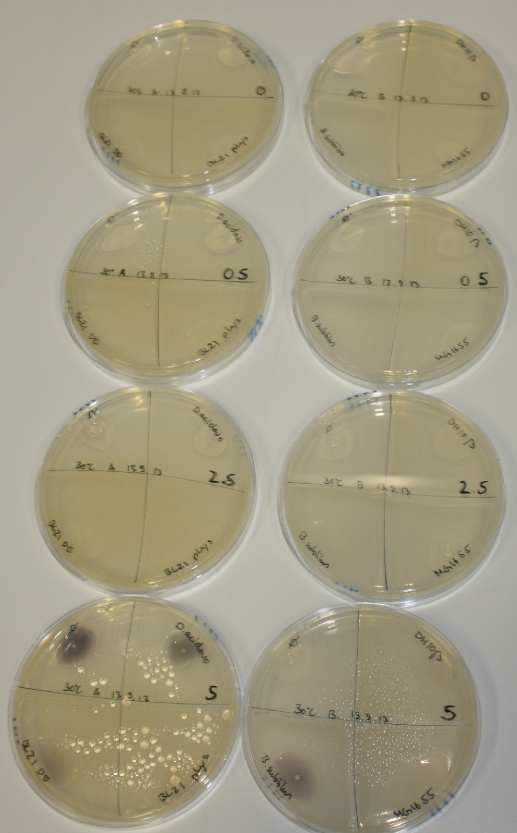
In order to optimize plates, growth time and incubation temperature of D.acidovorans as well as to evaluate of endogenous capability of possible target strains, various growth conditions and bacteria were tested.
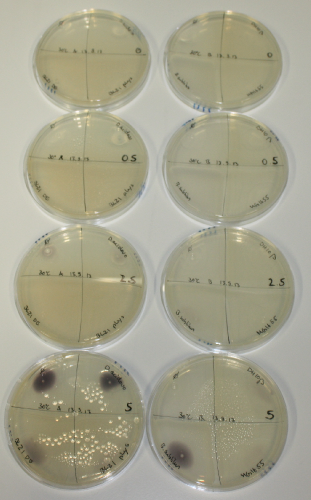
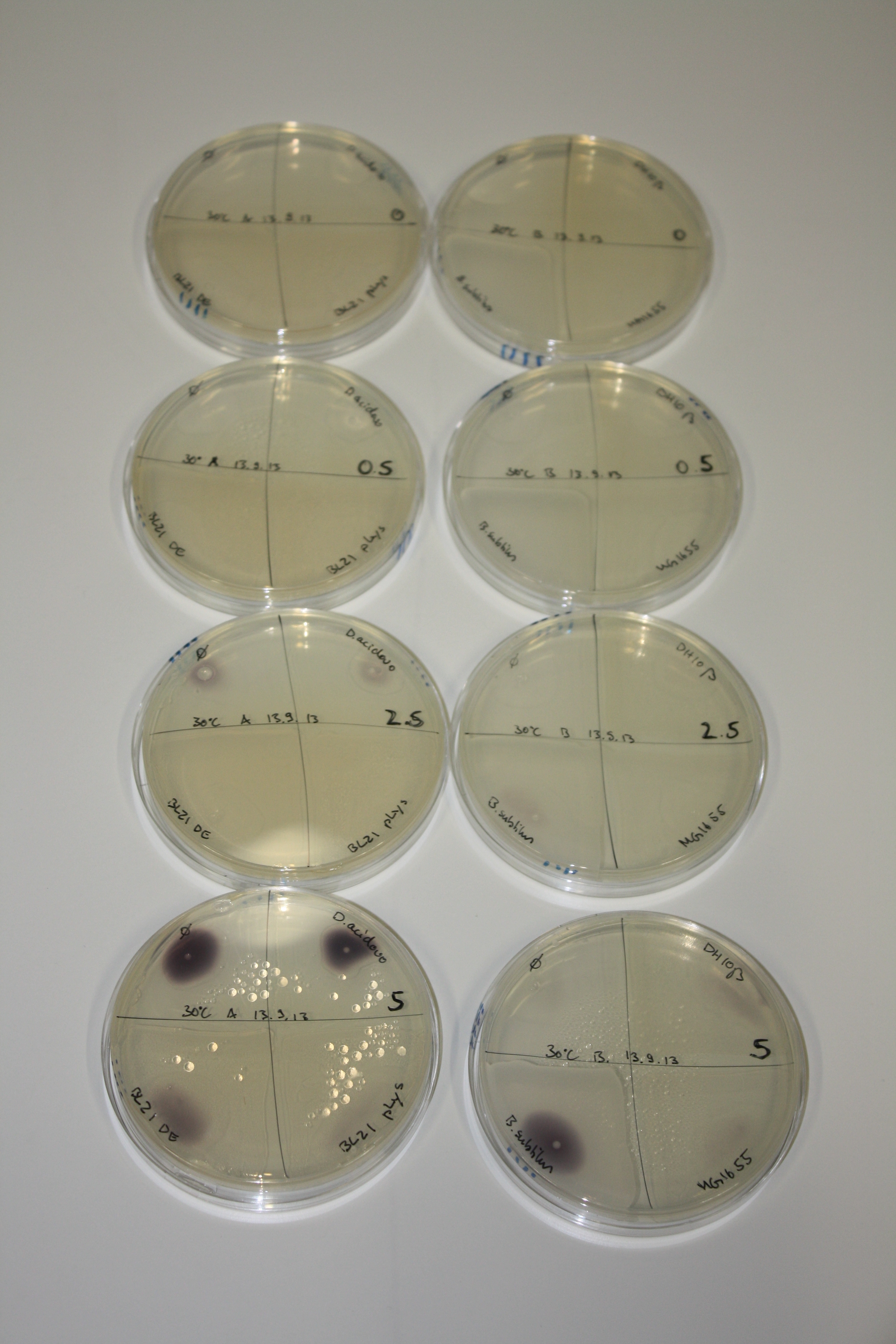
On Agar Plate following 3 Day Incubation at 30°C
- Media was chelex treated, agar added and autoclaved, before plates were poured.
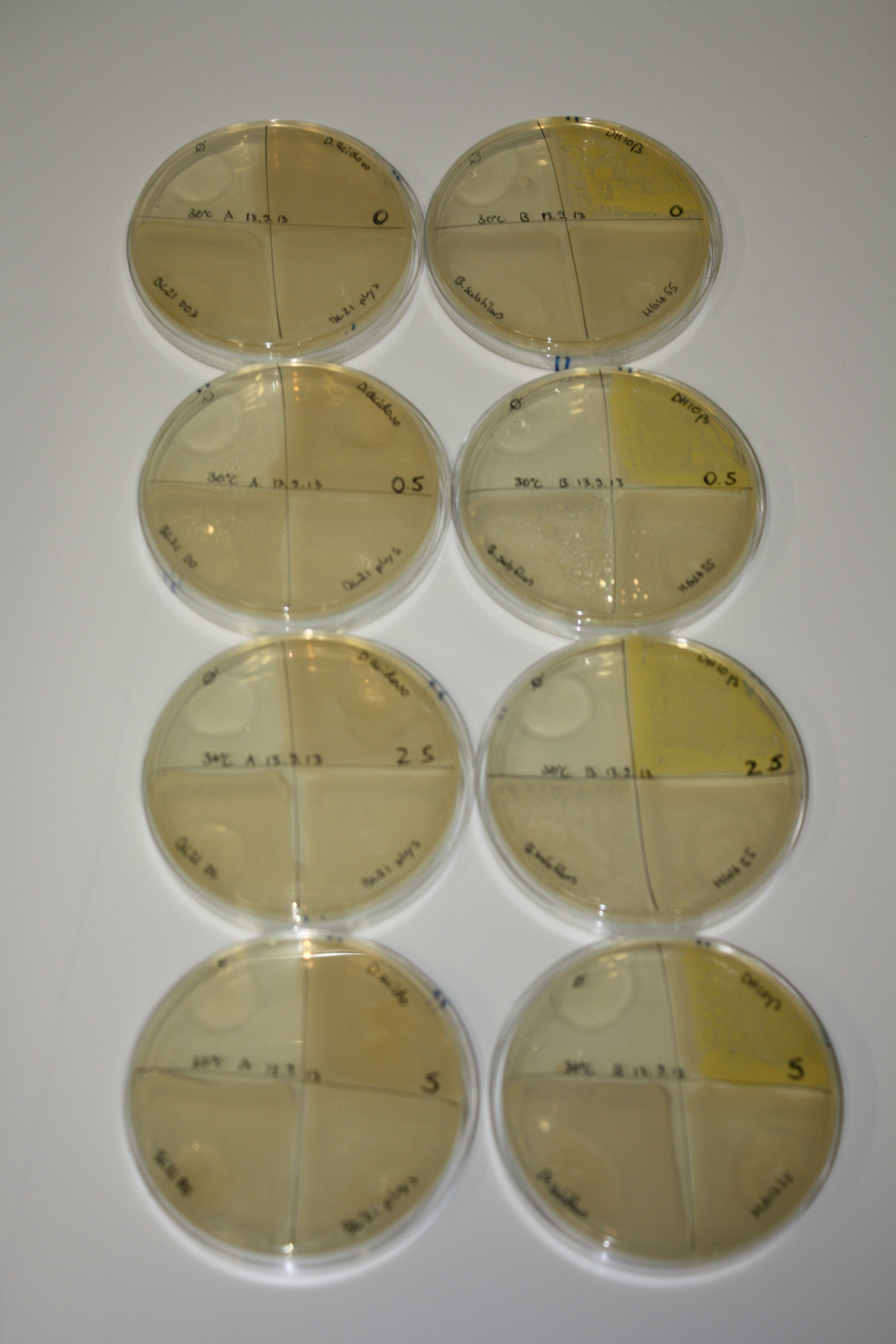
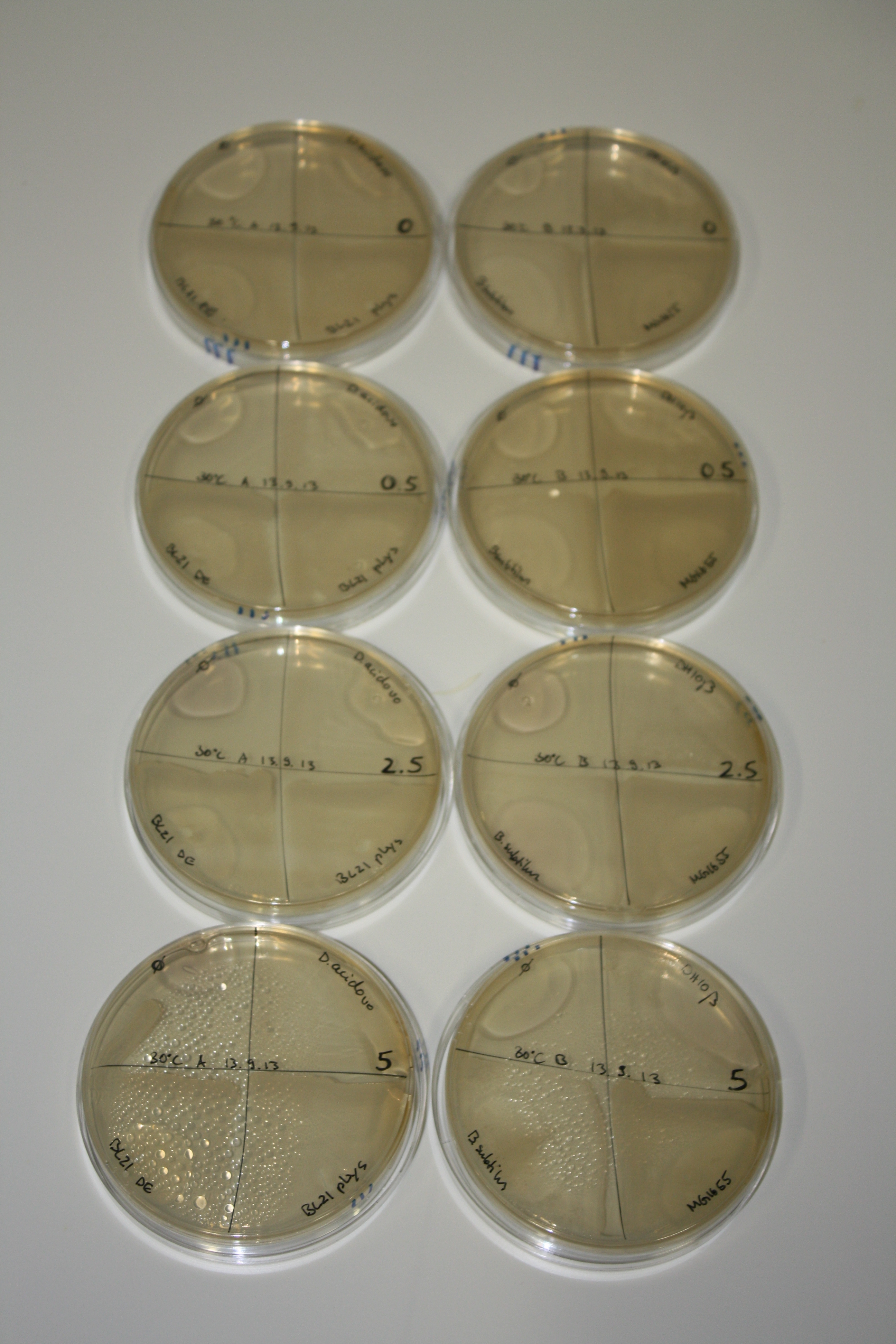
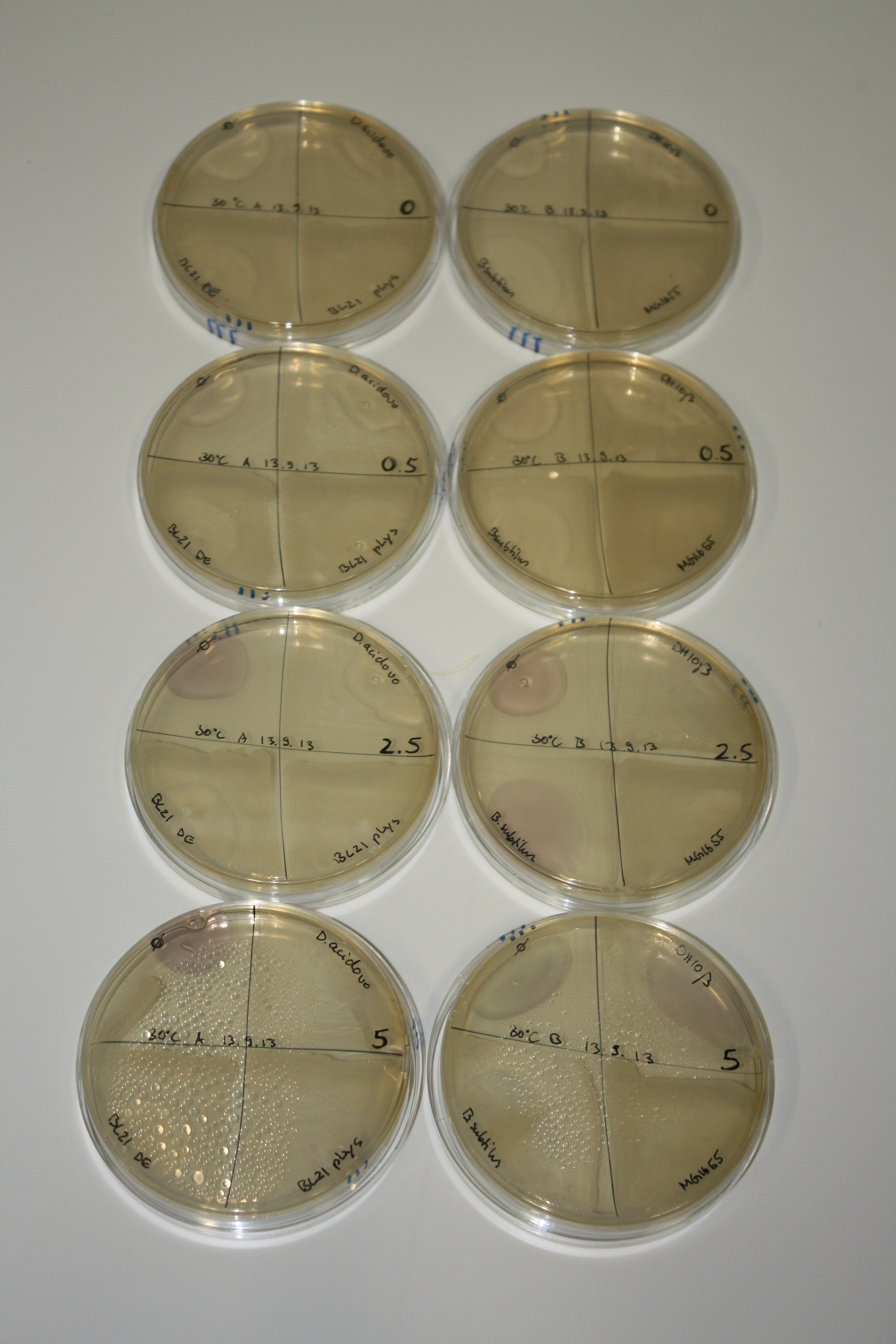
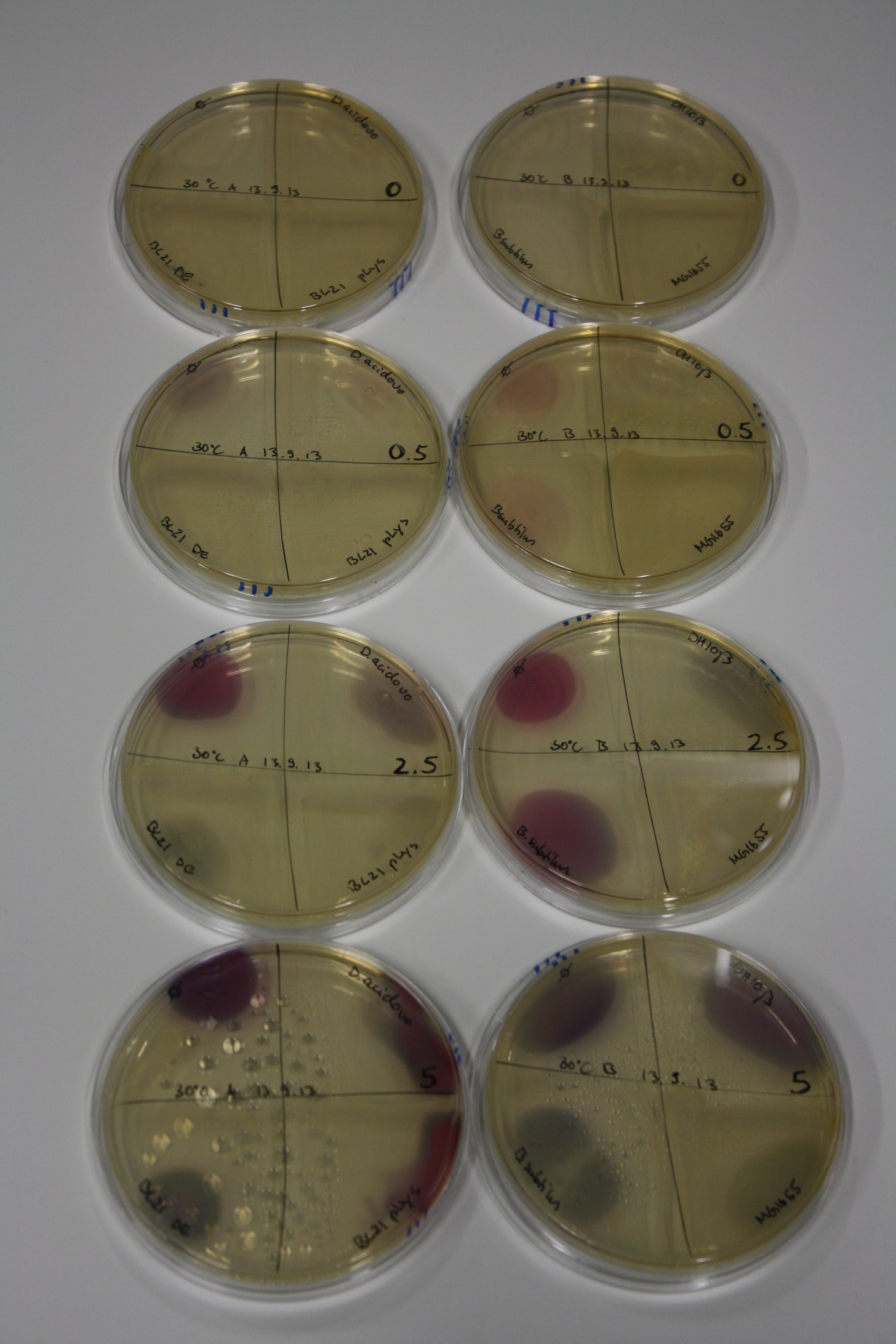
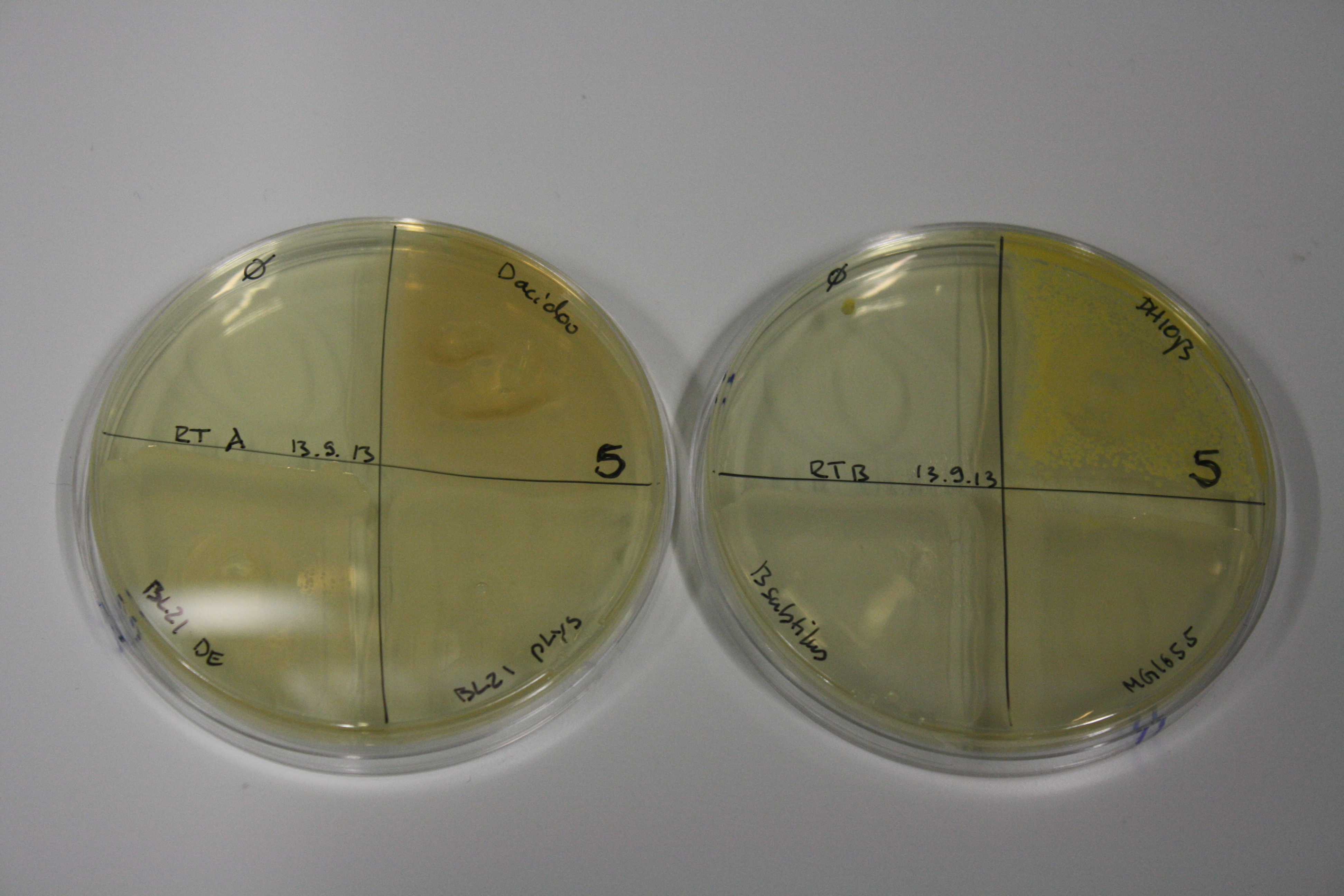
- On agar plates, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 and Bacillus subtilus were plated from liquid culture and grown for three days at 30 °C.
- 14.4 ml soft agar (0.5%) was mixed with gold chloride [100 g/l] as follows and 200 µl per quarter plate added:
| Final gold chloride concentration [mM] | Volume goldchloride stock [µl] |
|---|---|
| 0 | 0 |
| 0.5 | 2.7 |
| 2.5 | 13.5 |
| 5 | 27 |
Result
| Agar plate | Goldchloride [mM] | H2O | D.acidovorans | BL21 DE3 | BL21 pLys | H2O | DH10ß | B.circulans | MG1655 |
|---|---|---|---|---|---|---|---|---|---|
| ACM | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| ACM | 0.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| ACM | 2.5 | minor coloring | minor coloring | no reaction | no reaction | no reaction | no reaction | minor coloring | no reaction |
| ACM | 5 | strong coloring | strong coloring | intermediate coloring | minor coloring | minor coloring | minor coloring | minor coloring | minor coloring |
| LB | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| LB | 0.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| LB | 2.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| LB | 5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 0.5 | minor coloring | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 2.5 | strong coloring | minor coloring | no reaction | no reaction | strong coloring | minor coloring | strong coloring | no reaction |
| M9 | 5 | strong coloring | minor coloring | no reaction | no reaction | minor coloring | minor coloring | minor coloring | no reaction |
Unfortunately, the streaked E.coli DH10ß were contaminated (obvious only on LB plates) and can threfore not be analyzed.
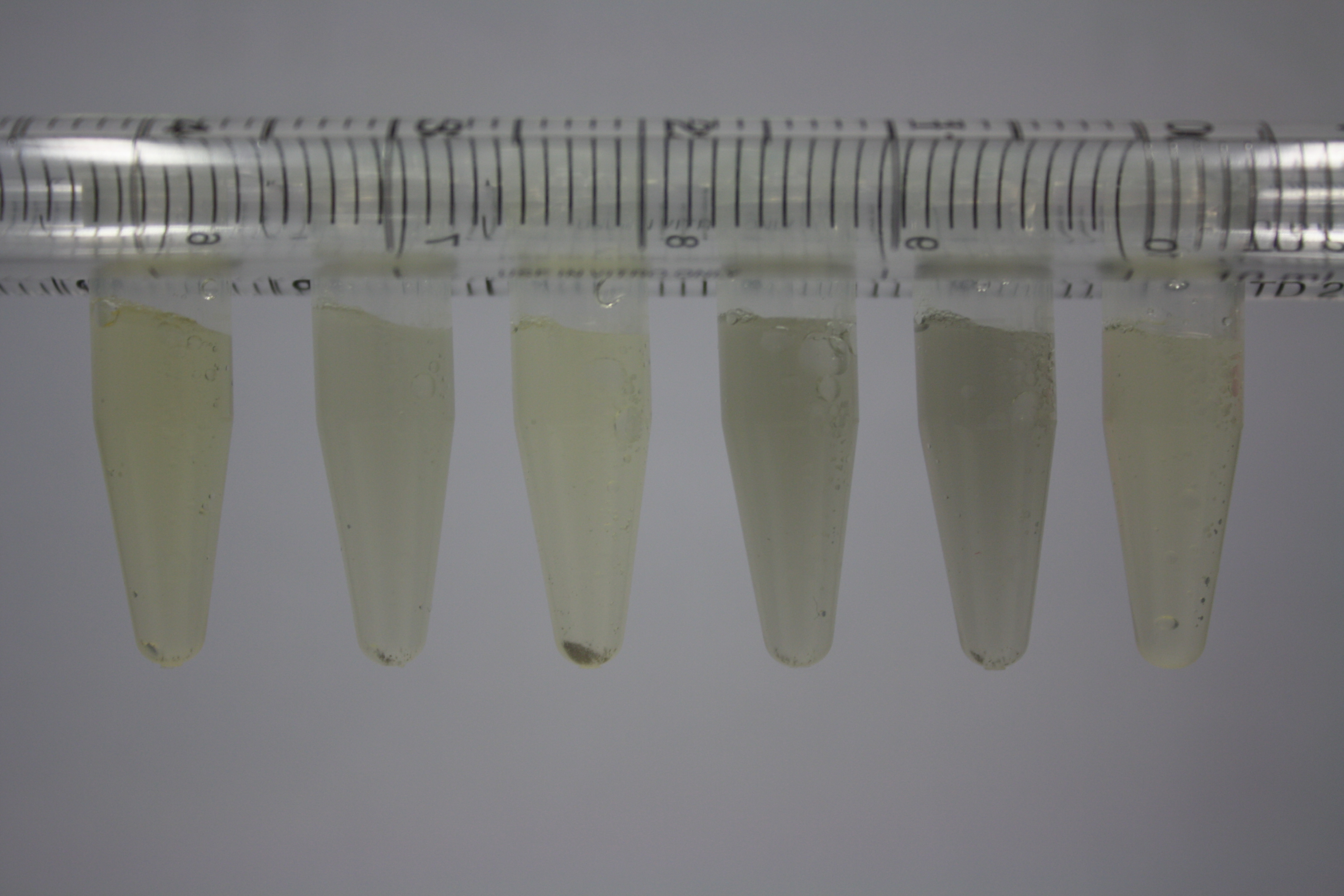
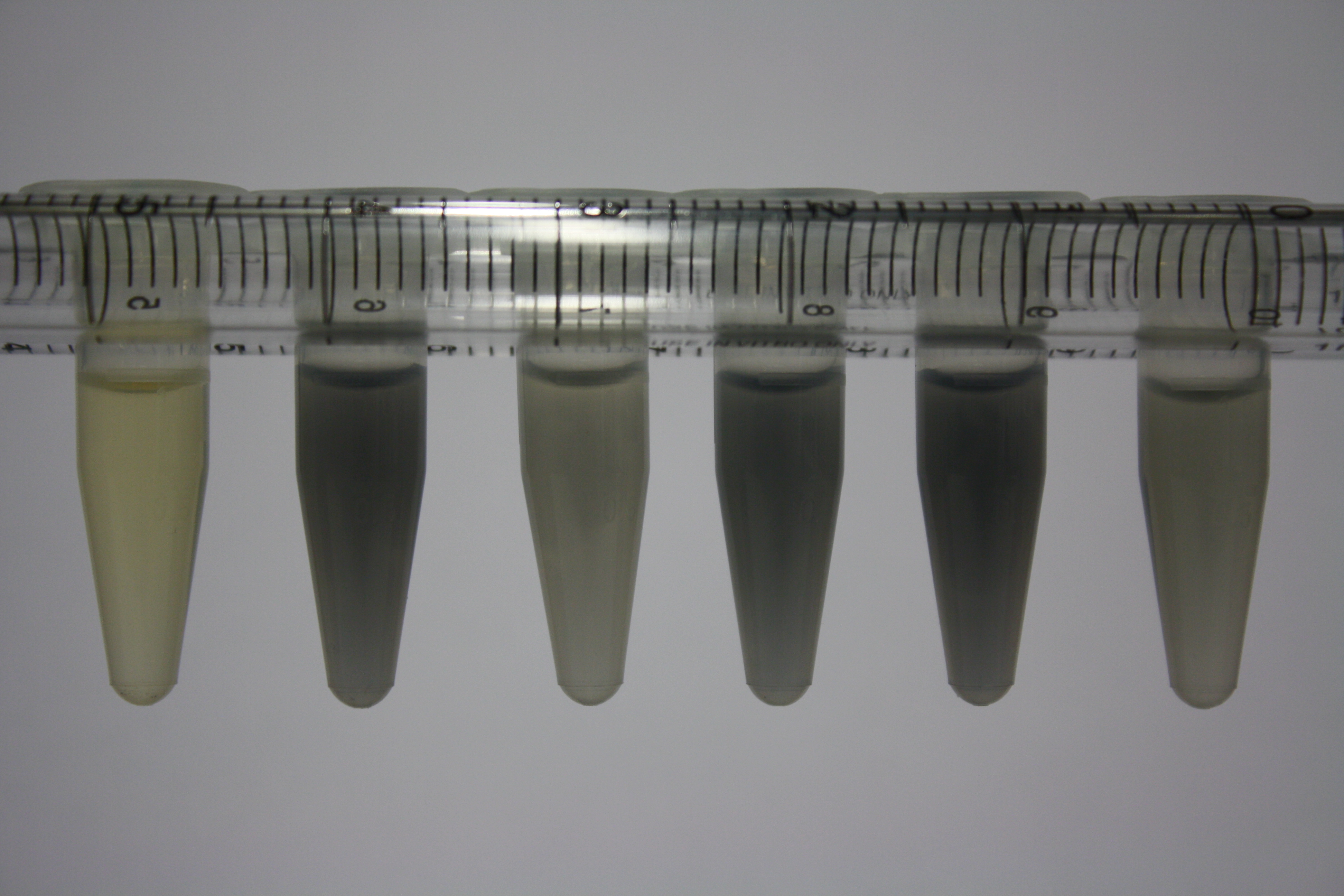
On ACM plates following 30°C incubation, D.acidovorans, B.circulans, E.coli BL21 DE3 as well as the negative control next to D.acidovorans, but not the one on plate B, showed immediate purple-black coloring indicating formation of gold nanoparticles at 5 mM gold chloride. At 2.5 mM, D.acidovorans and the neighbouring negative control as well as B.circulans showed some coloring. E.coli BL21 pLys and MG1655 only showed minor coloring at 5 mM. At lower concentrations, no gold precipitation was observed.
- => E.coli BL21 pLys and MG1655 are promissing candidates for expression since they show the least background activity.
- => Optimal gold chloride concentration lies between 2.5 and 5 mM.
- => Delftibactin can diffuse through agar.
On LB plates following 30°C incubation, none of the tested bacteria showed any reaction.
- => LB is not suitable for expression of NRPs.
- => What is missing in LB that does not allow metall precipitating activity?
On M9 plates following 30°C incubation (which were mistakenly autoclaved after addition of glucose), D.acidovorans hardly grew. At 5 mM and 2.5 mM gold chloride, there is heavy background activity on the negative controls. Additionally, B.circulans shows strong - D.acidovorans minor formation of nanoparticles.
- => M9 is not suitable for gold precipitation experiments.
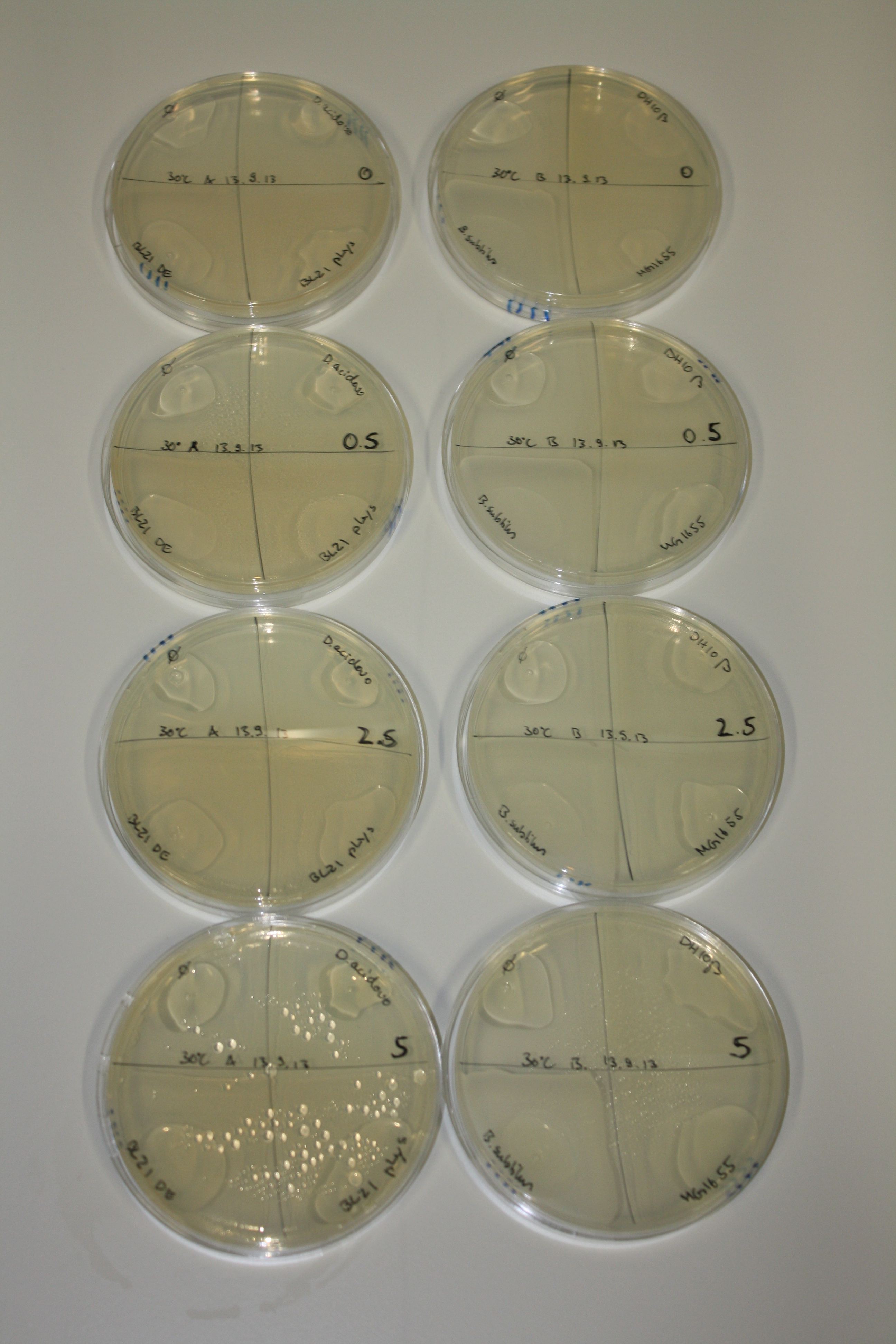
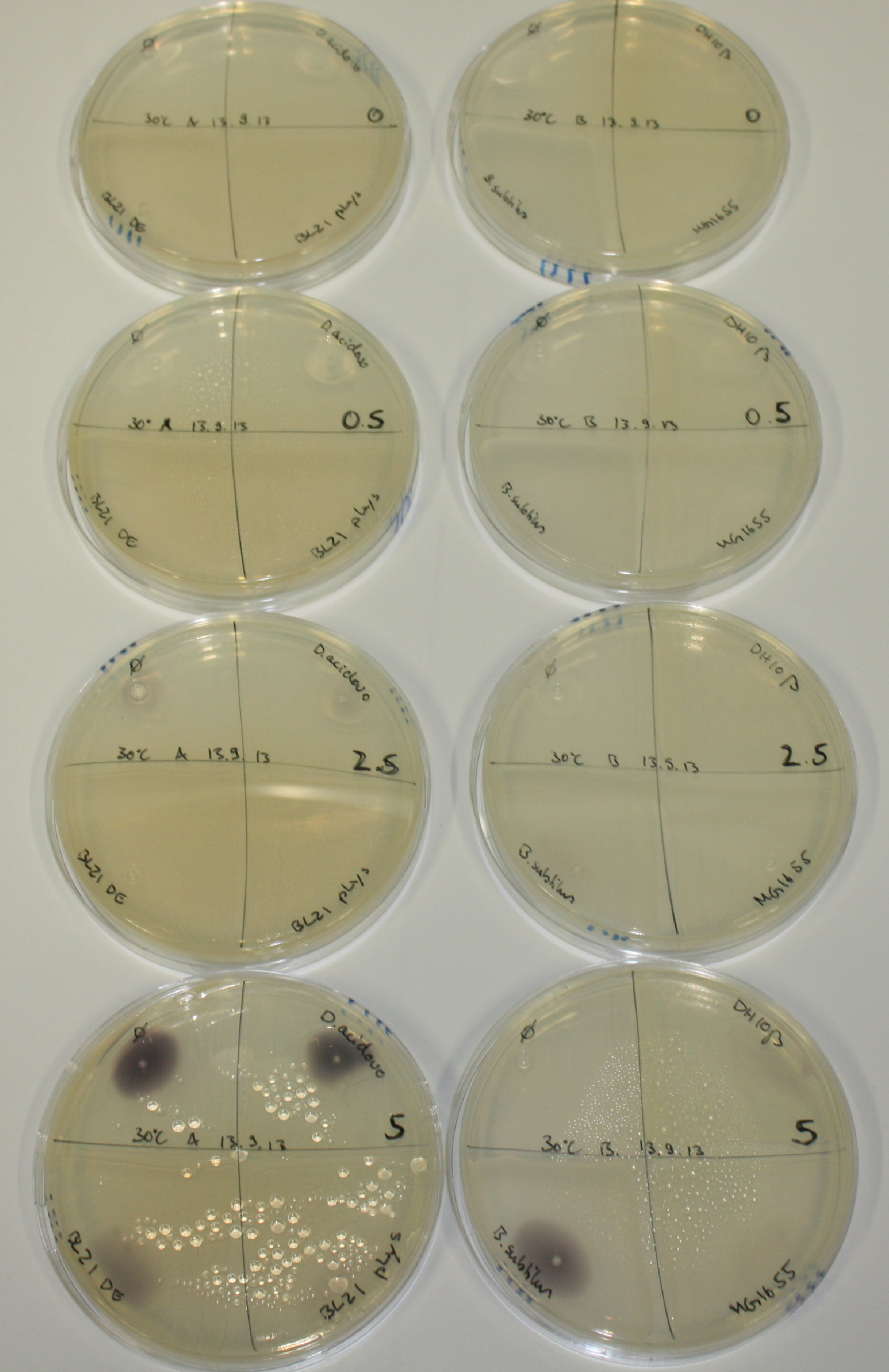
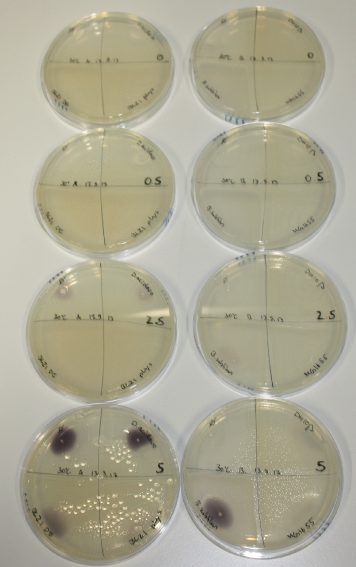
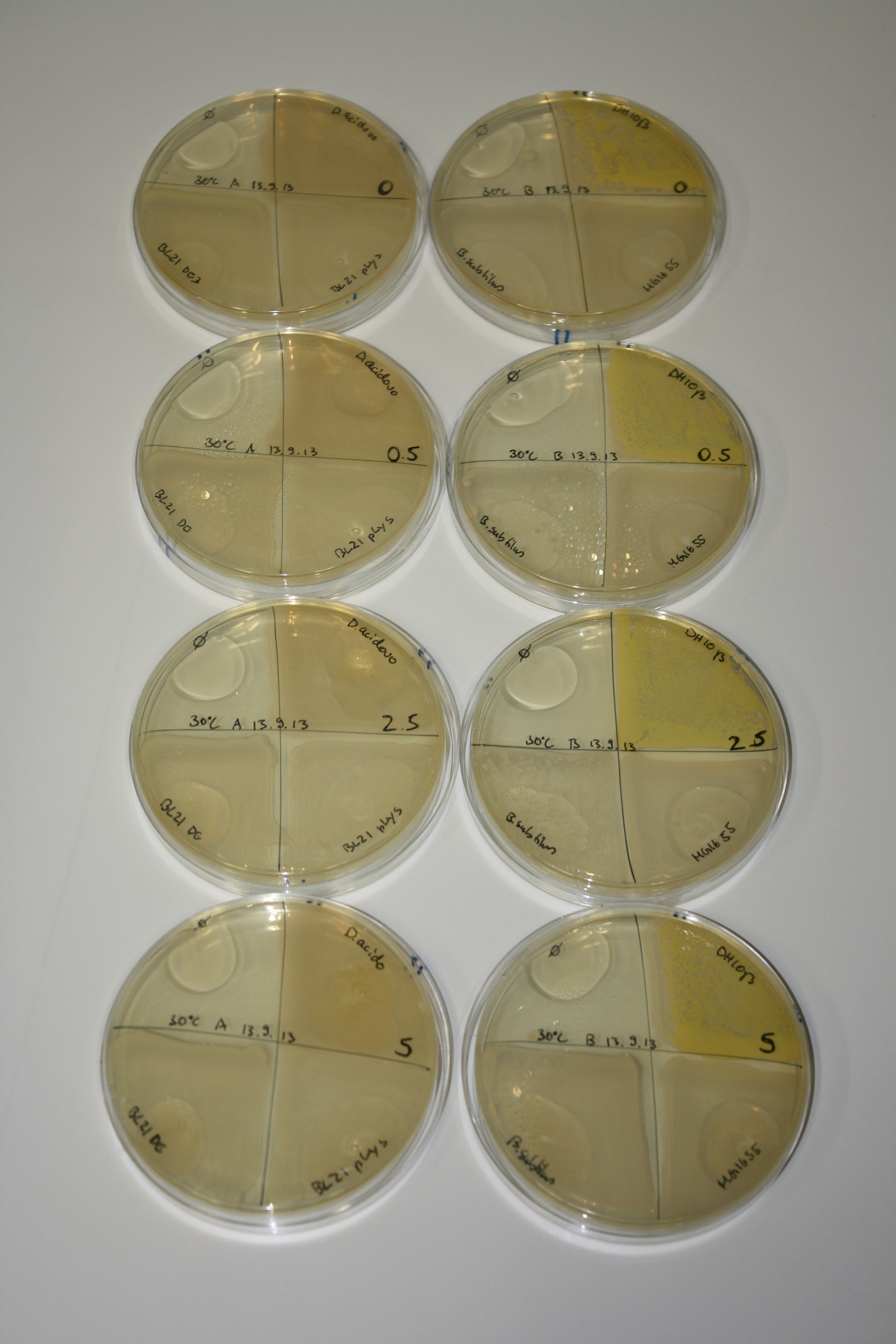
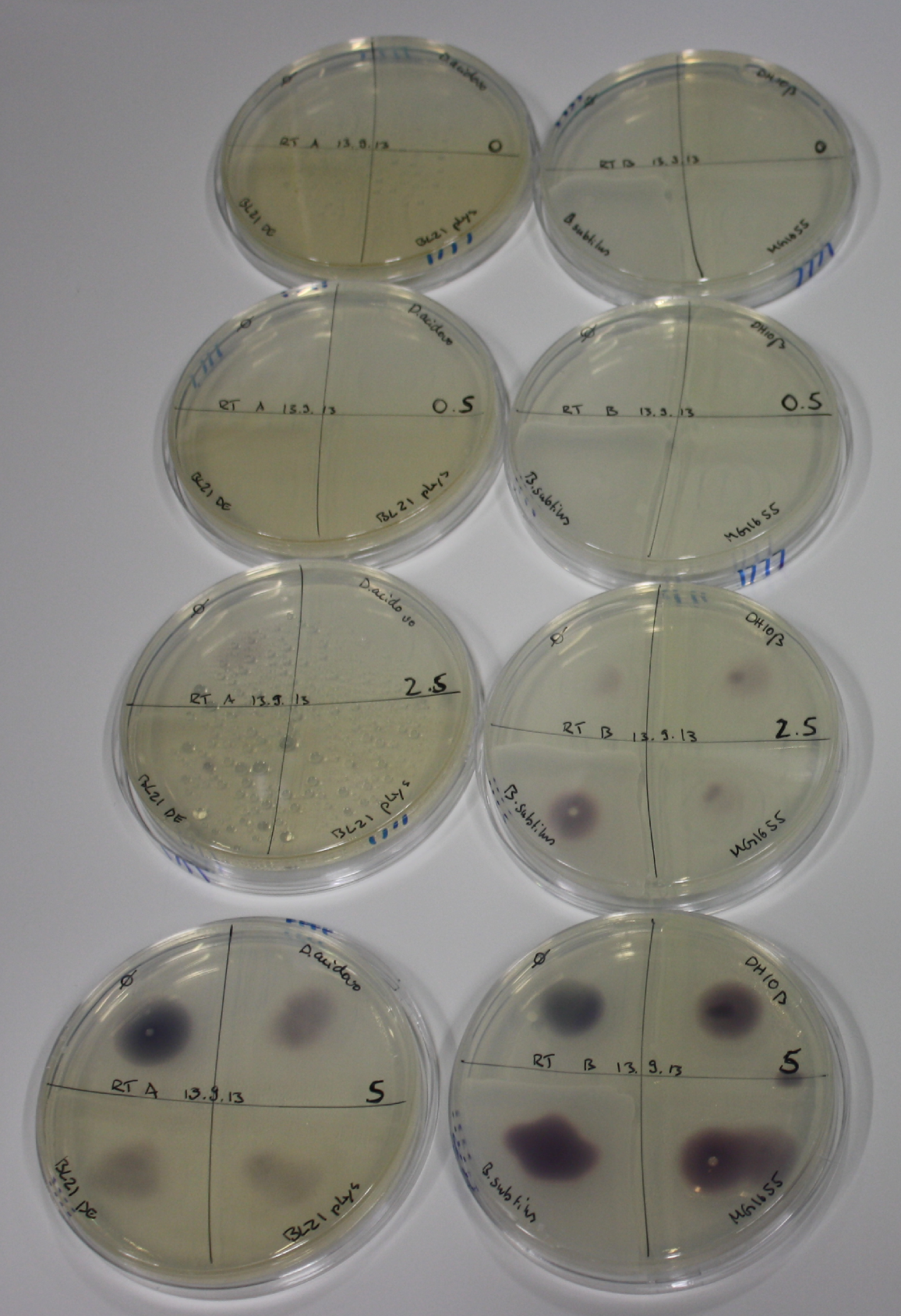
On Agar Plate following 3 Day Incubation at RT
- Media was chelex treated, agar added and autoclaved, before plates were poured.
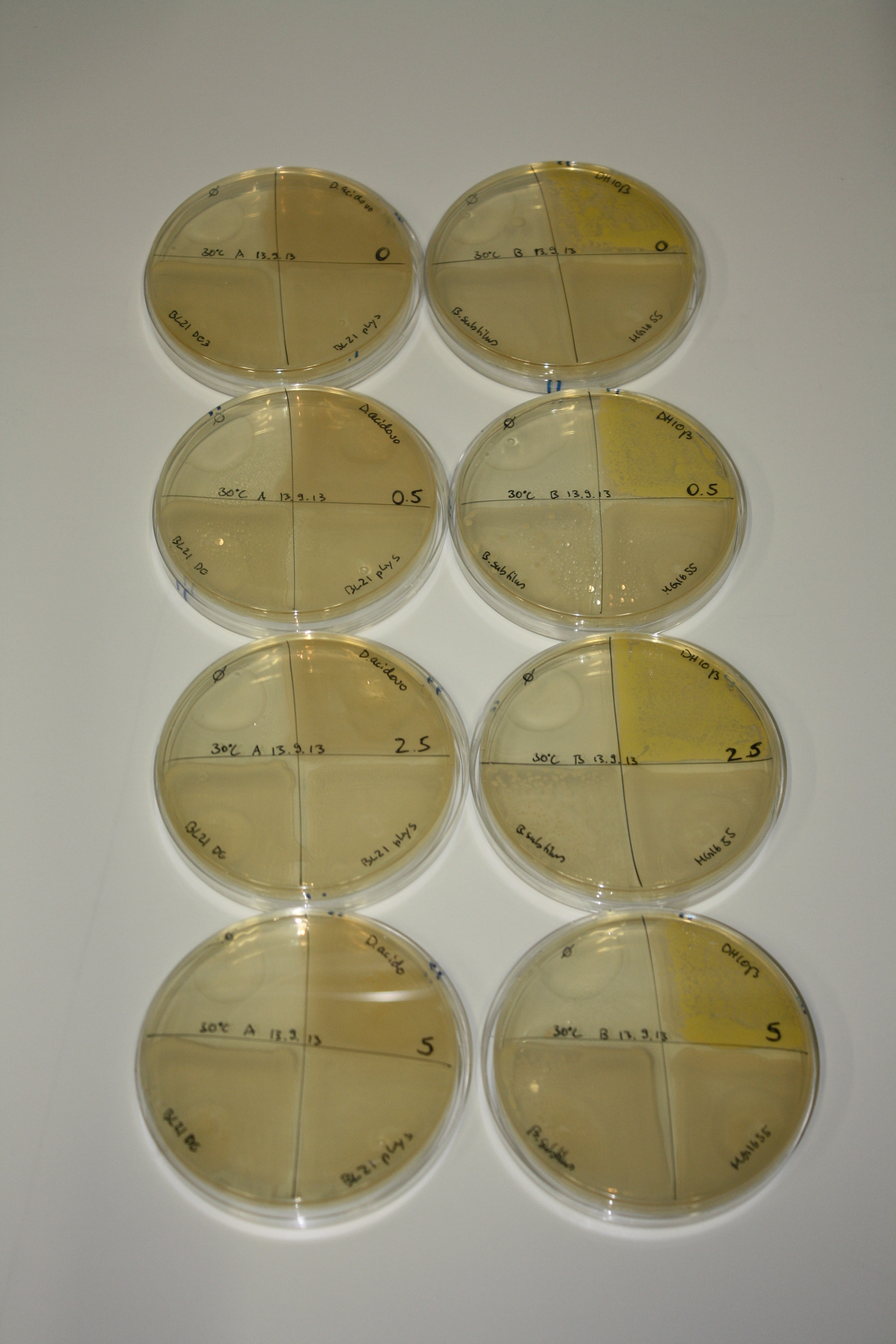
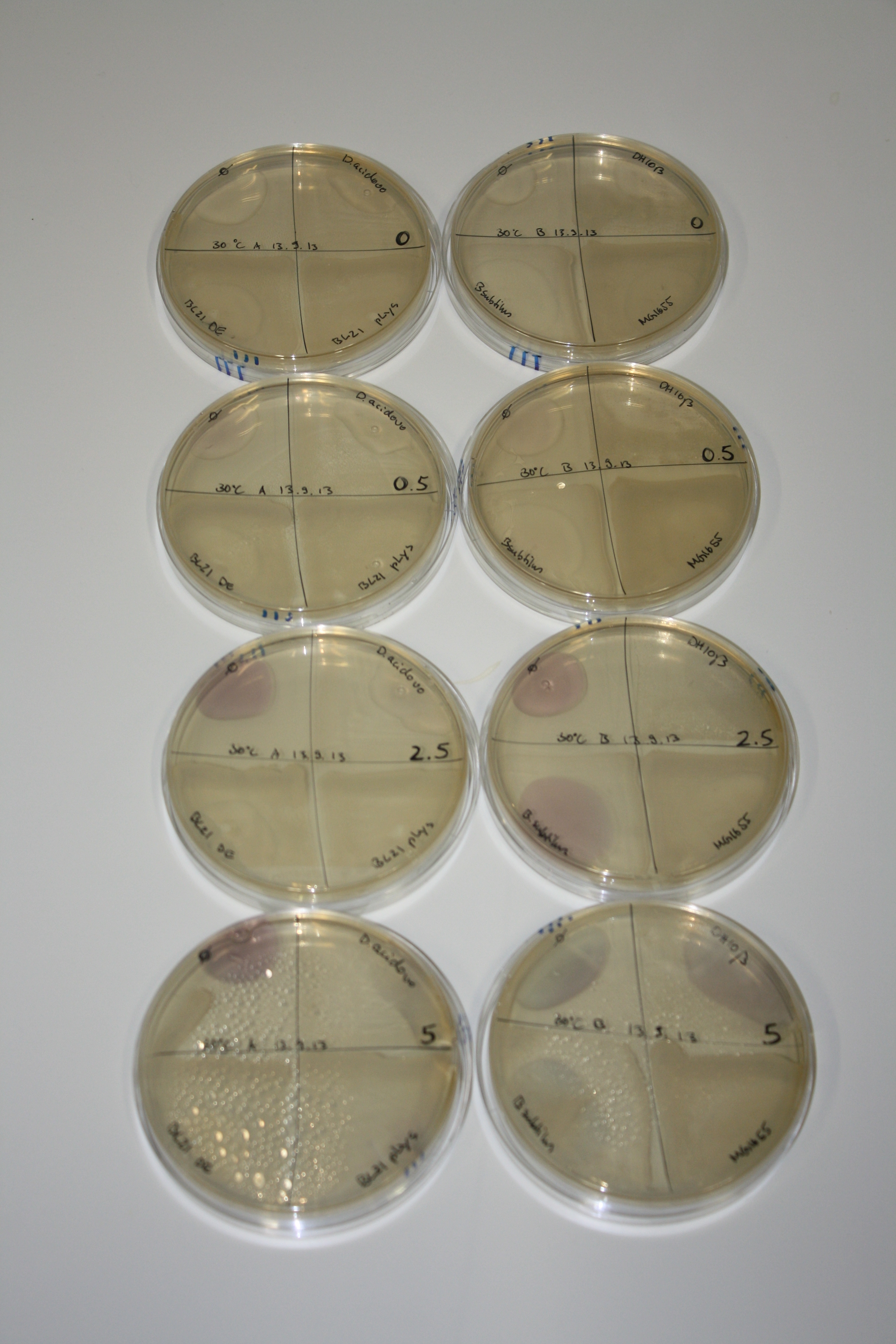
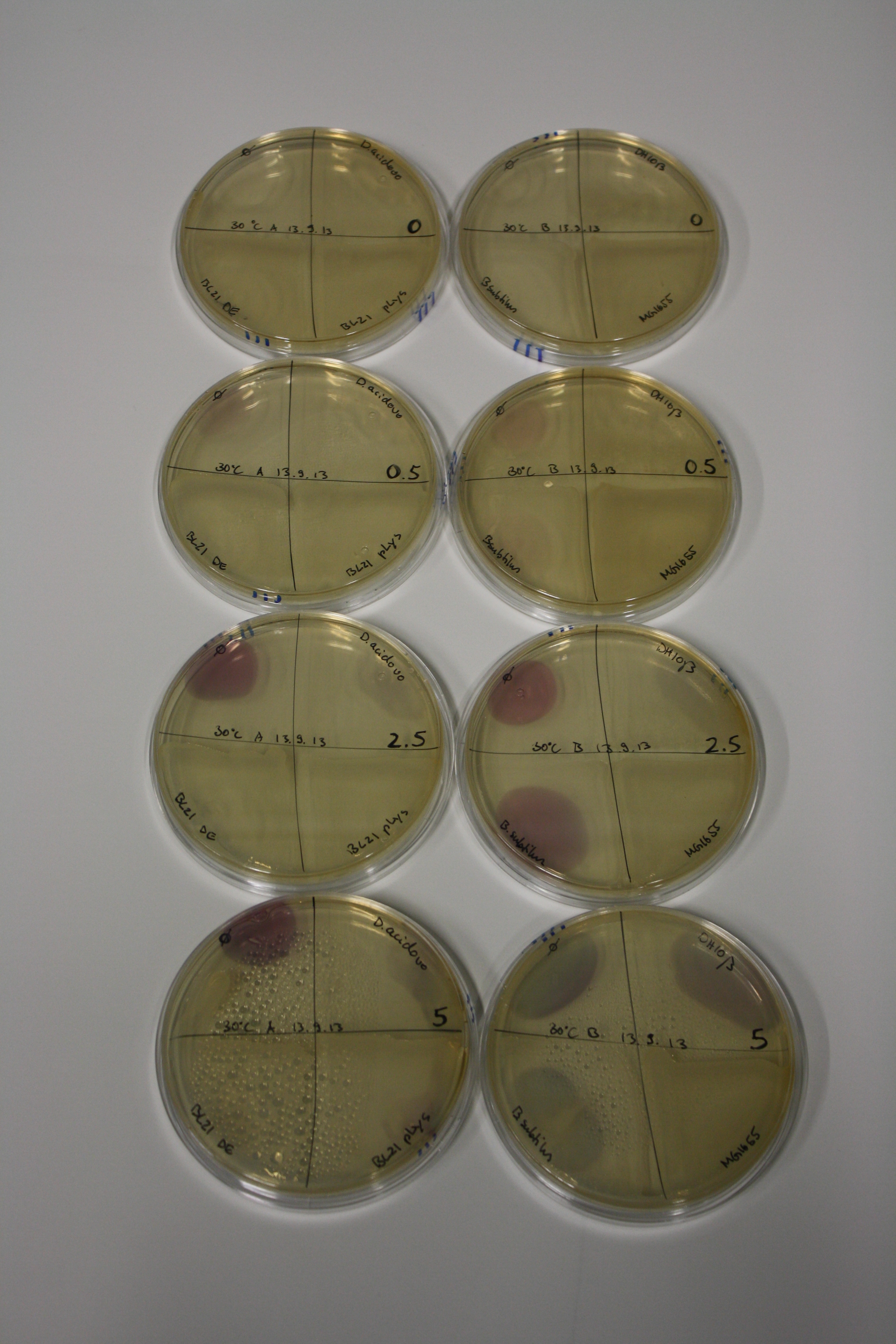
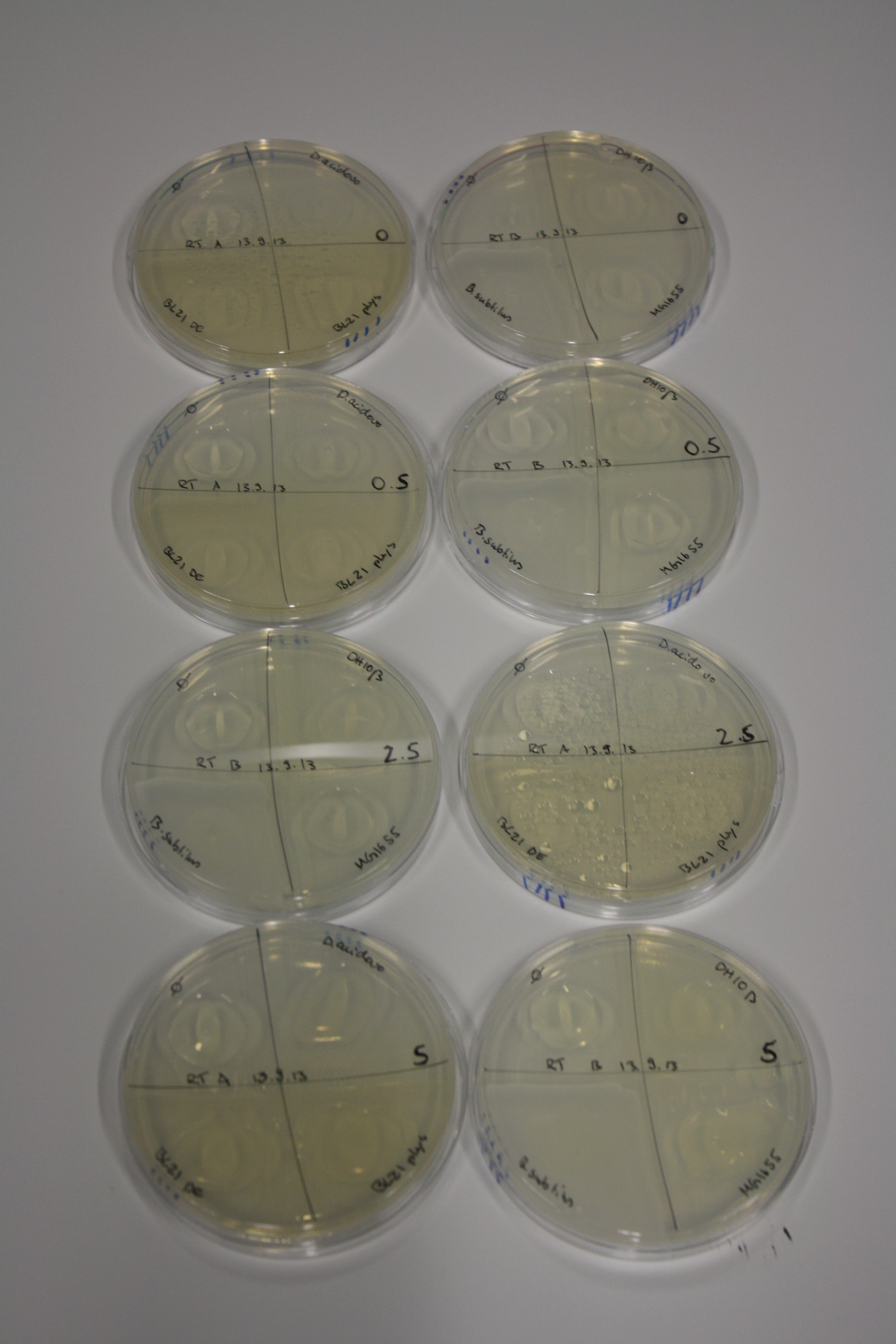
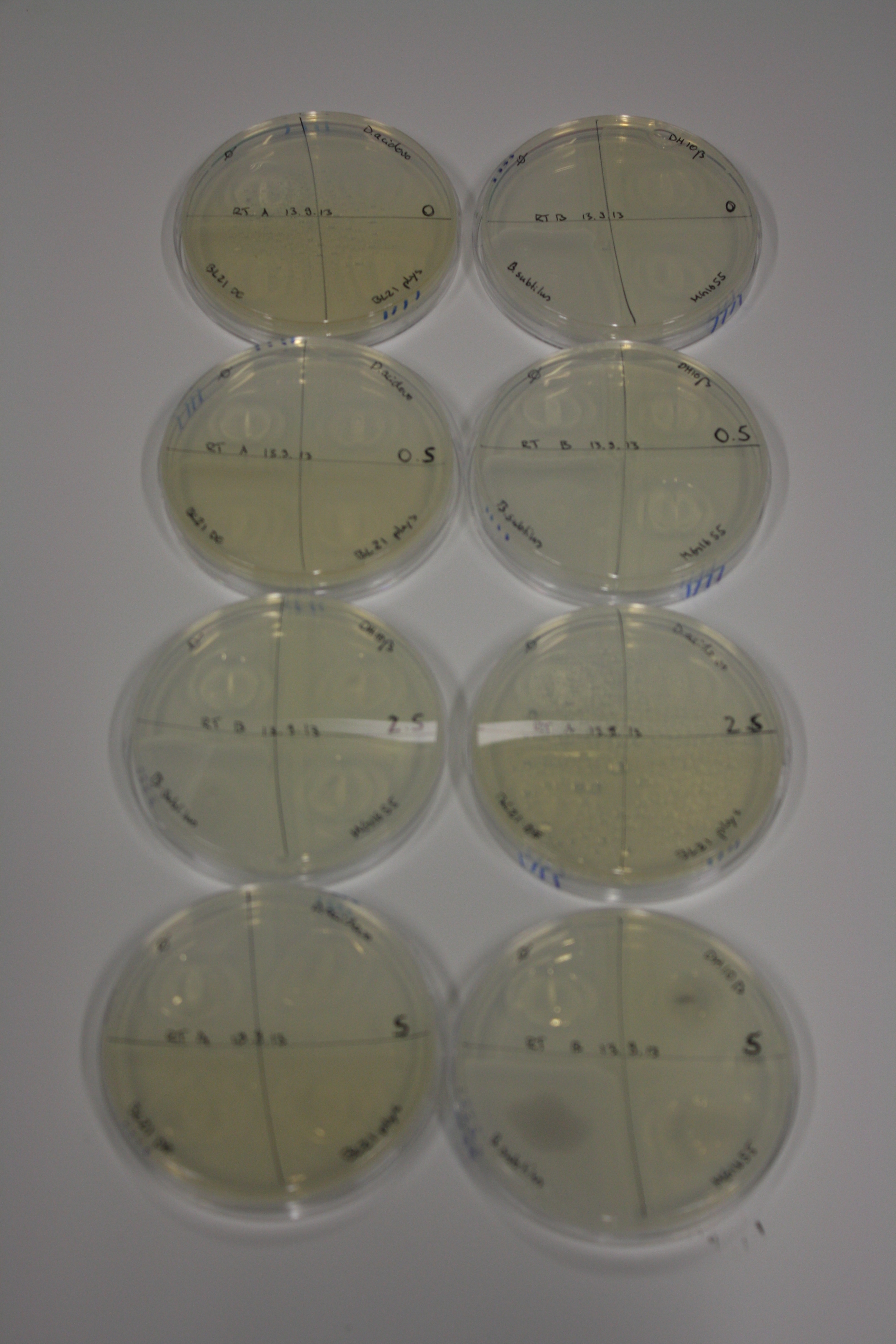
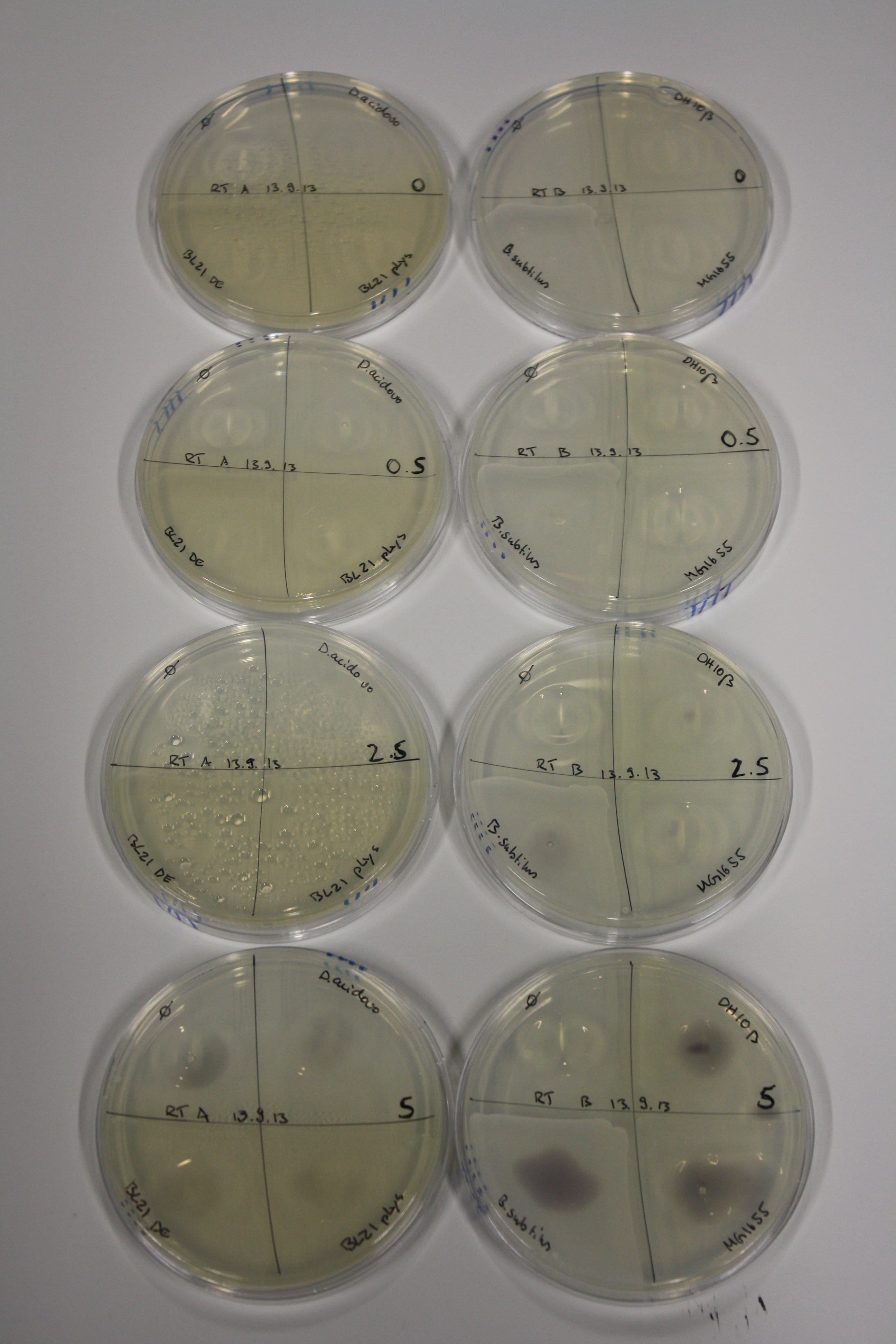
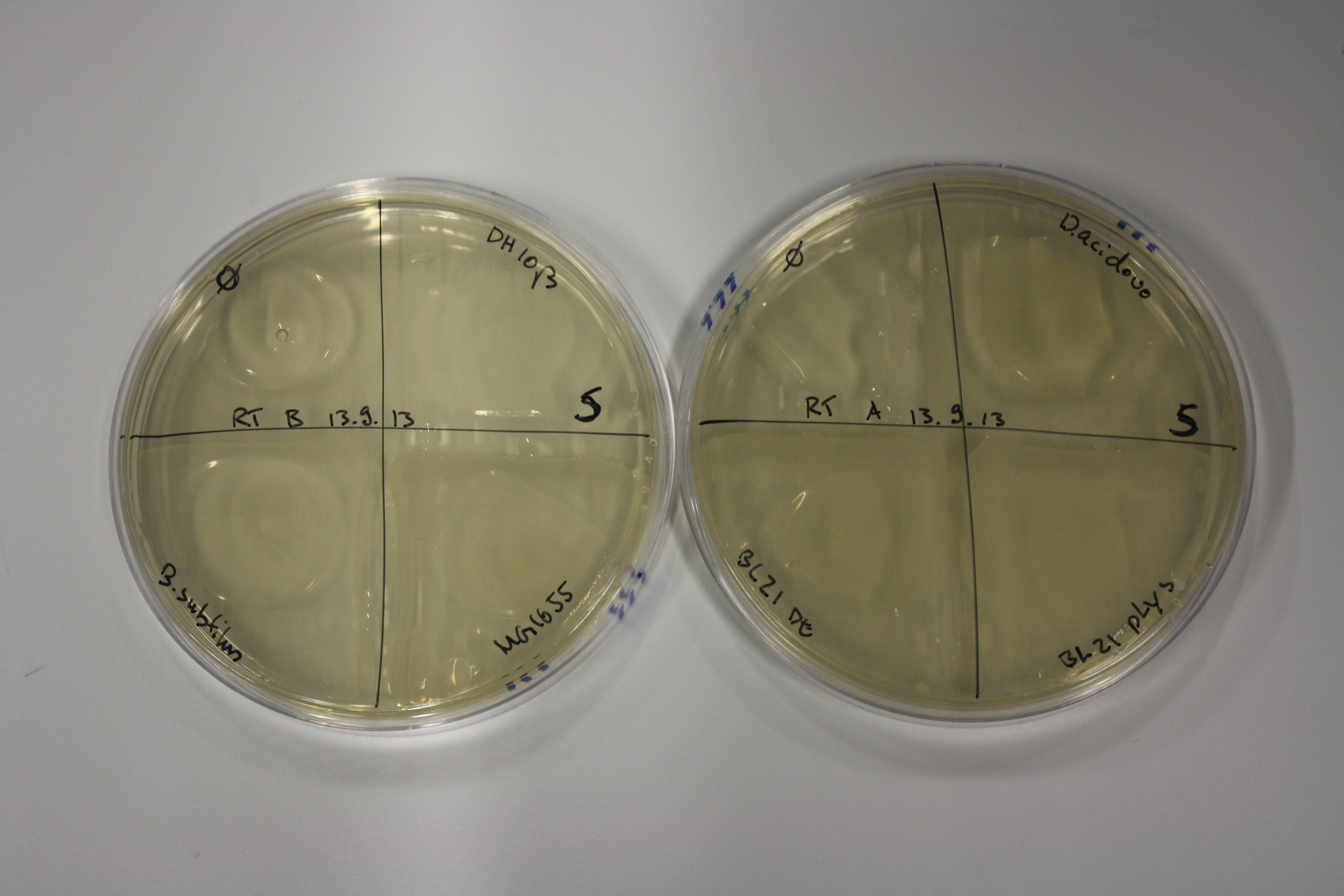
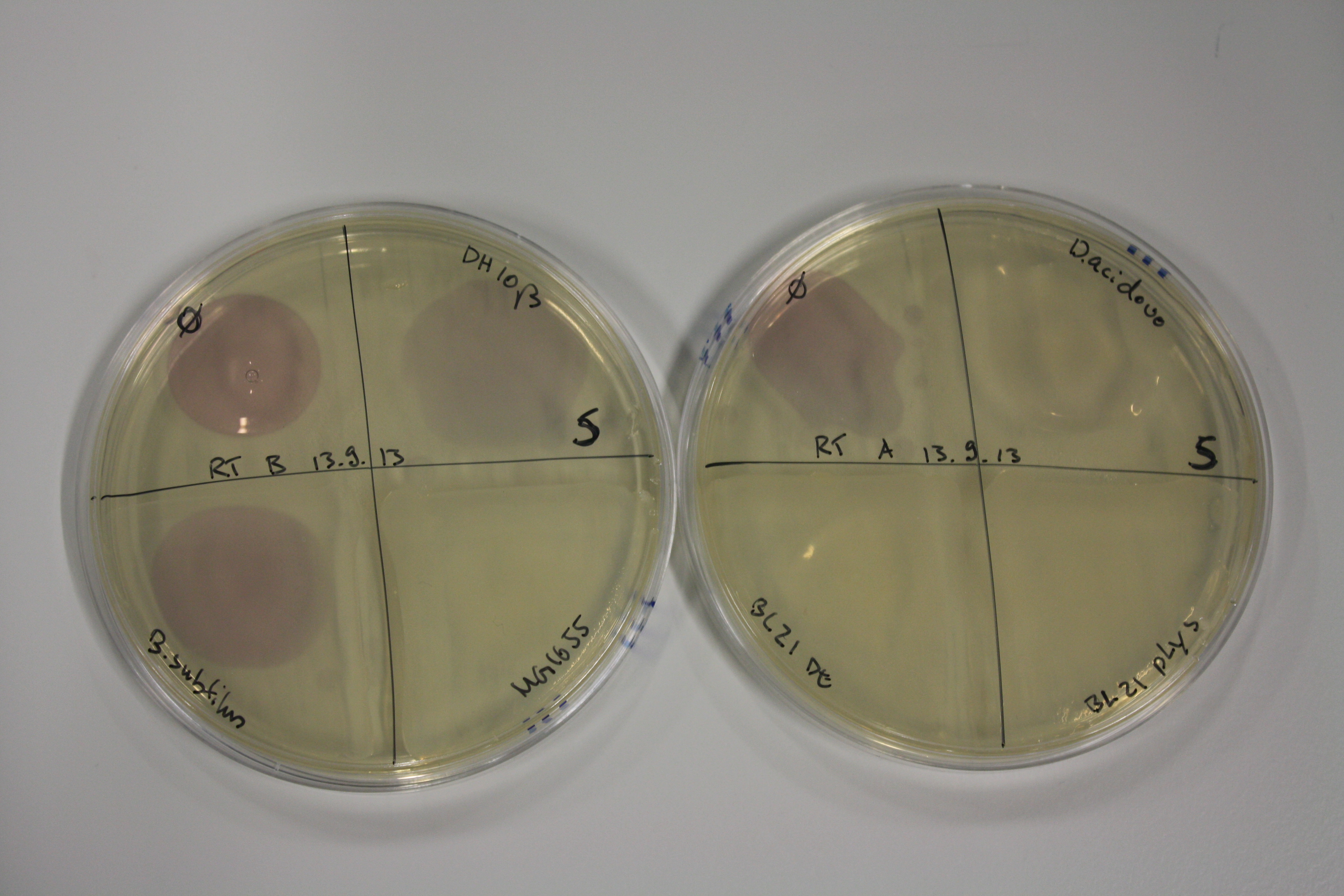
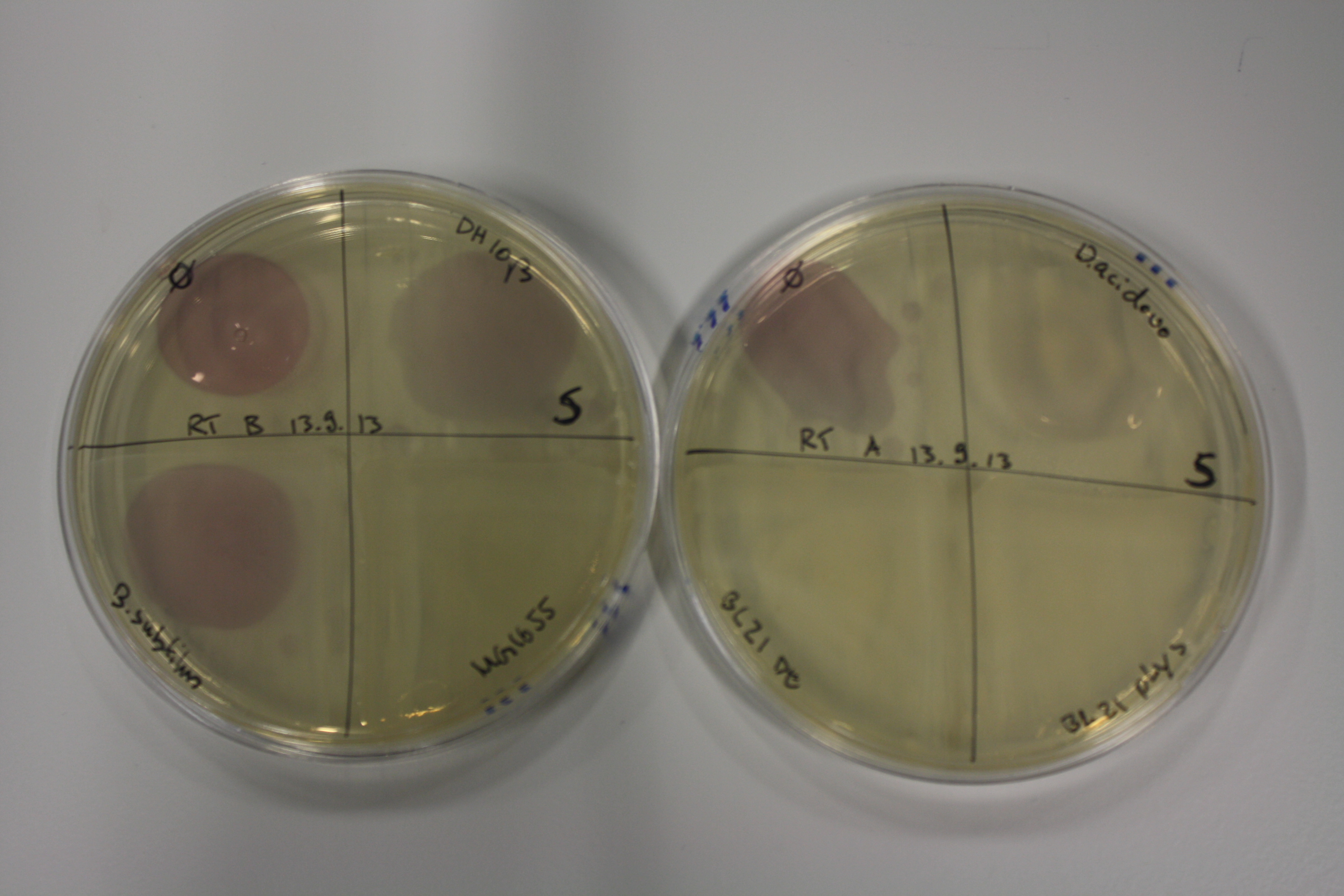
- On agar plates, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 and Bacillus subtilus were plated from liquid culture and grown for three days at RT.
- 14.4 ml soft agar (0.5%) was mixed with gold chloride [100 g/l] as follows and 200 µl per quarter plate added:
| Final gold chloride concentration [mM] | Volume goldchloride stock [µl] |
|---|---|
| 0 | 0 |
| 0.5 | 2.7 |
| 2.5 | 13.5 |
| 5 | 27 |
Result
| Agar plate | Goldchloride [mM] | H2O | D.acidovorans | BL21 DE3 | BL21 pLys | H2O | DH10ß | B.circulans | MG1655 |
|---|---|---|---|---|---|---|---|---|---|
| ACM | 0 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| ACM | 0.5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| ACM | 2.5 | no reaction | no reaction | no reaction | no reaction | minor coloring | minor coloring | intermediate coloring | minor coloring |
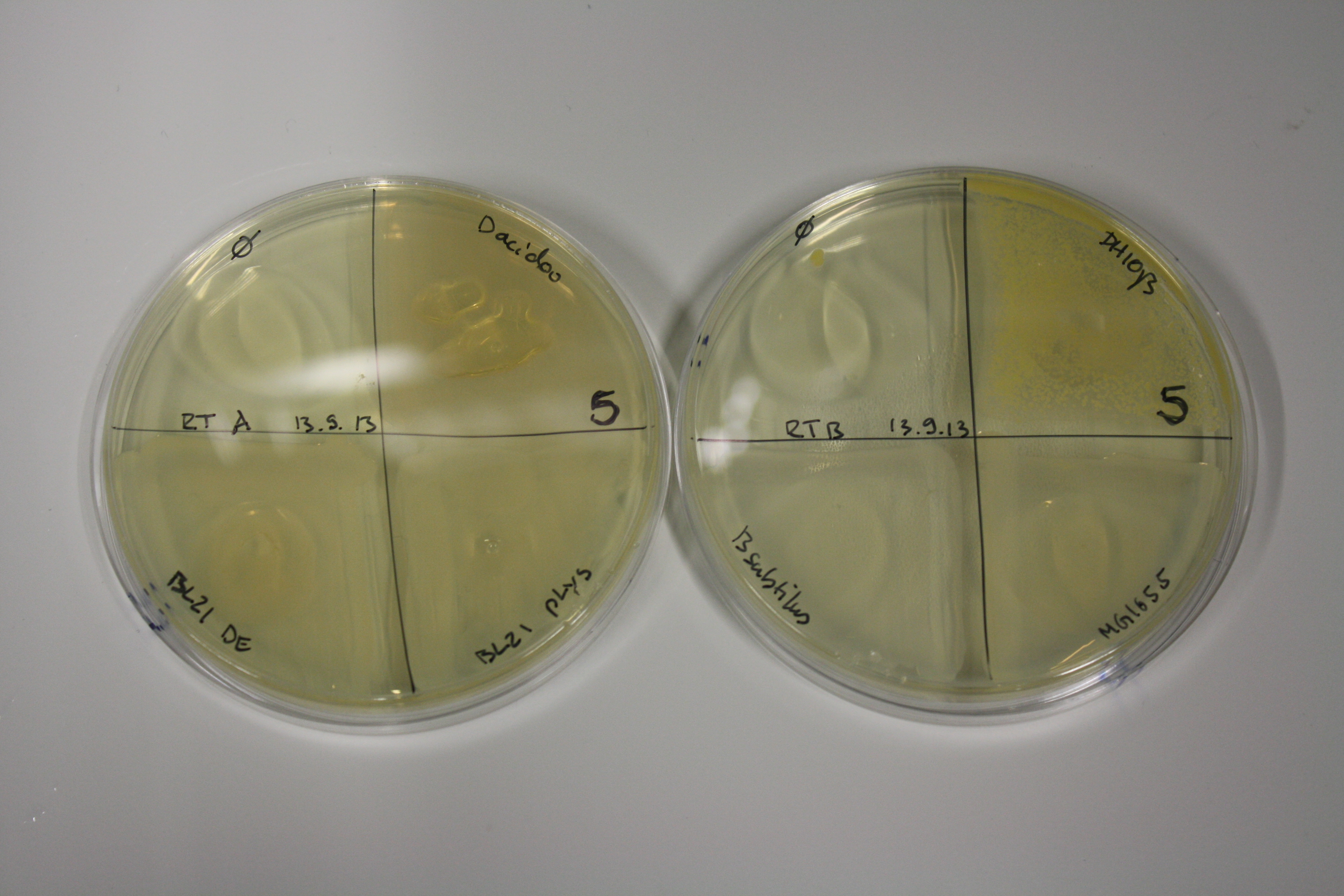
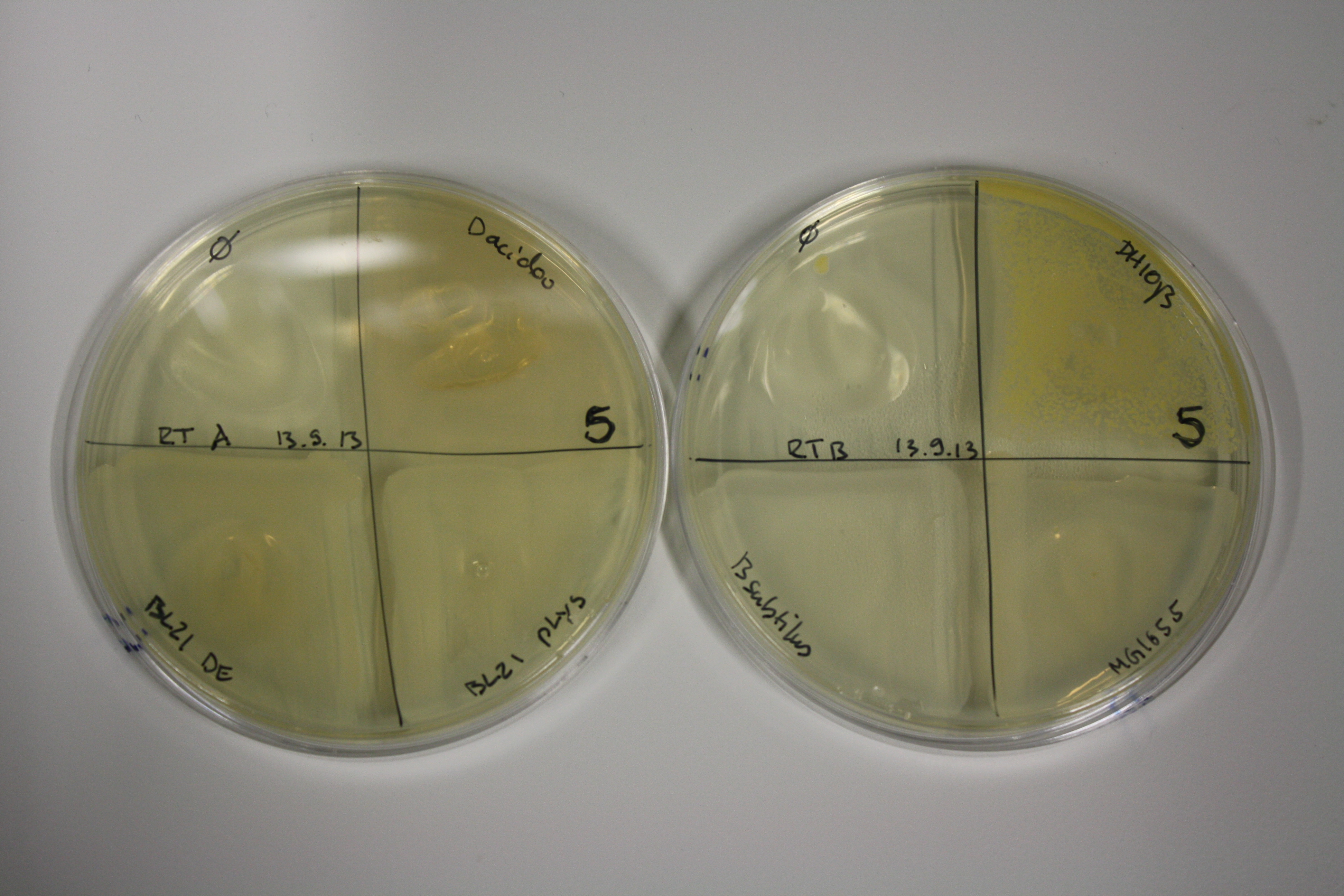
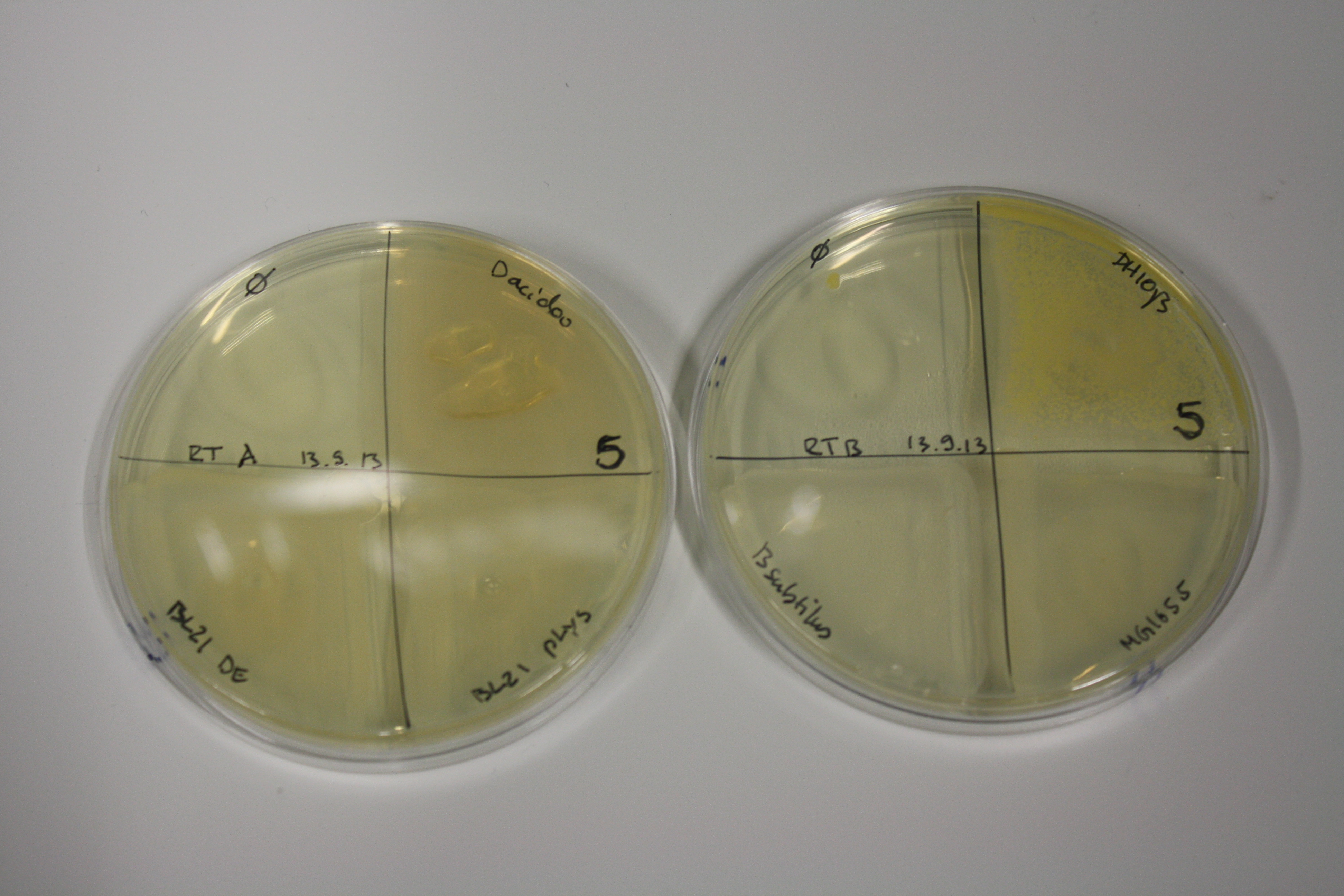
| ACM | 5 | strong coloring | strong coloring | minor coloring | minor coloring | intermediate coloring | strong coloring | strong coloring | strong coloring |
| LB | 5 | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction | no reaction |
| M9 | 5 | strong coloring | strong coloring | no reaction | no reaction | strong coloring | strong coloring | strong coloring | no reaction |
On ACM plates following RT incubation, D.acidovorans and the neighbouring negative control, B.circulans and E.coli MG1655 show minor immediate coloring at 5 mM. At lower concentrations, no activity can be observed.
- => There is less Delftibactin expression in D.acidovorans at lower temperatures.
- => E.coli BL21 pLys remains a promissing candidate for expression since they show the least background activity.
On LB plates following RT incubation, there is again nothing to see.
On M9 plates following RT incubation, there is again high background at negative controls as well as intermediate coloring for D.acidovorans and B.circulans.
We will therefore analyze gold precipitation capacities of supernatants of D.acidovorans, E.coli DH10ß, BL21 DE3, BL21 pLys and MG1655 in ACM liquid medium following 48 and 72 h.
In ACM Medium following 2 Day Incubation at 30°C
- In 5 ml ACM medium, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 and pure medium were inocculated and grown for two days at 30°C.
- 1 ml supernatant was mixed with gold chloride [100 g/l] as follows:
| Final gold chloride concentration [µg/ml] | Volume goldchloride stock [µl] |
|---|---|
| 0.6 | 6 |
Result
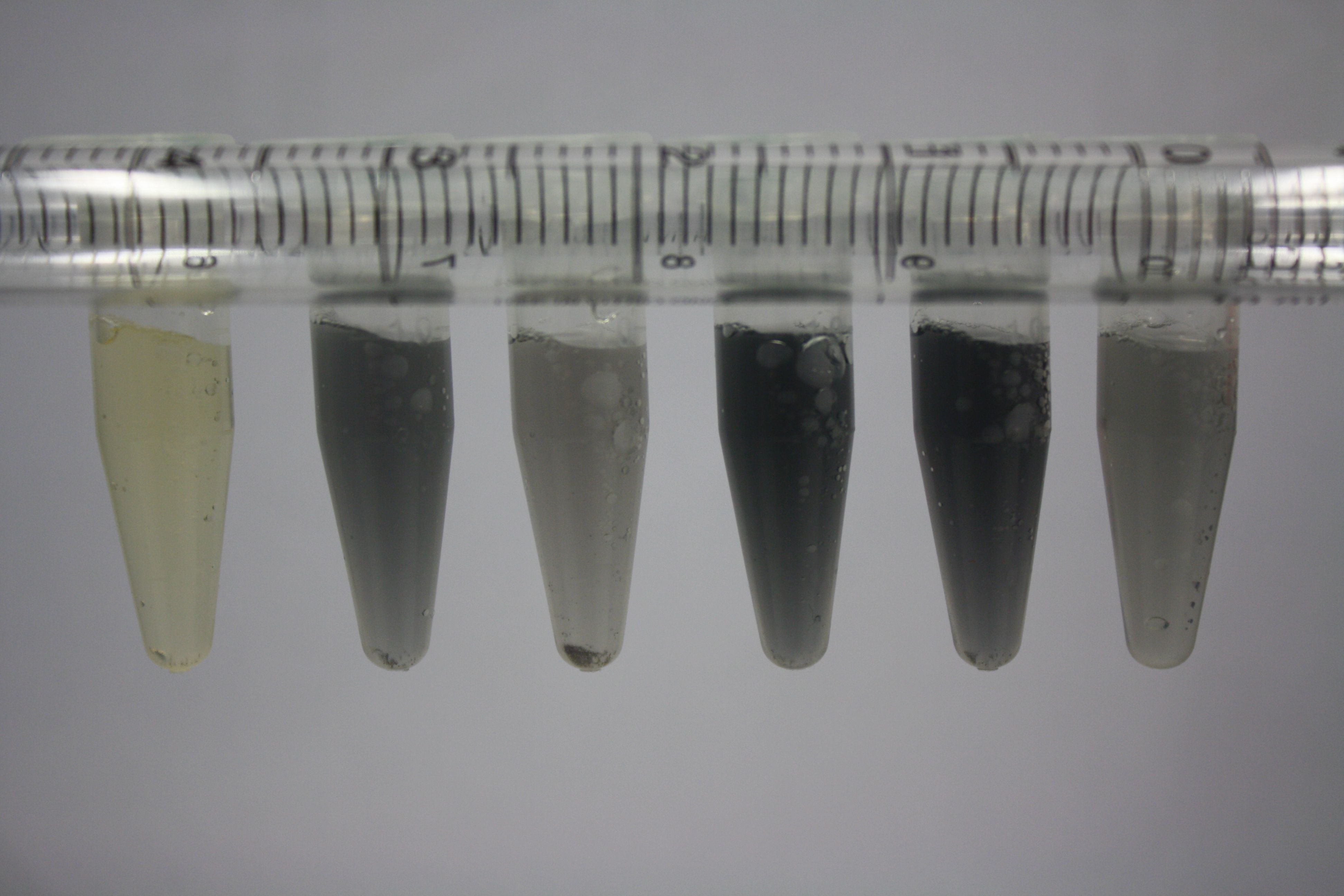
Following addition of gold chloride solution, E.coli DH10ß, Bl21 DE3, and BL21 pLys showed rapid purple-black coloring indicating gold nanoparticle formation. They exceed the capacity of D.acidovorans, which showed gold precipitation slightly faster than E.coli MG1655. The ACM did not show any background activity.
We will extend the incubation time of the cultures further to evaluate differences, possibly due to accumulation of Delftibactin in the medium.
In ACM Medium following 3 day Incubation at 30°C
- In 5 ml ACM medium, D.acidovorans, E.coli DH10ß, Bl21 DE3, and BL21 pLys as well as MG1655 were inocculated and grown for three days at 30°C.
- 1 ml supernatant was mixed with gold chloride [100 g/l] as follows:
| Final gold chloride concentration [µg/ml] | Volume goldchloride stock [µl] |
|---|---|
| 0.6 | 6 |
Result
Again, E. coli DH10ß, Bl21 DE3, and BL21 pLys showed rapid purple-black coloring exceeding the capacity of D.acidovorans and E. coli MG1655. These results are highly inconclusive and the experiment has to be repeated.
Purification of Delftibactin
Purification
- Grew 1 L of D. acidovorans SPH1 for 24h in ACM media at 30°C. (2x 500 mL ACM 15 mL about 3 days old D. acidovorans culture in ACM)
- Centrifugation of culture at 3750 rpm for 30 min
- Retrieved supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 400 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
Testing Purified Delftibactin
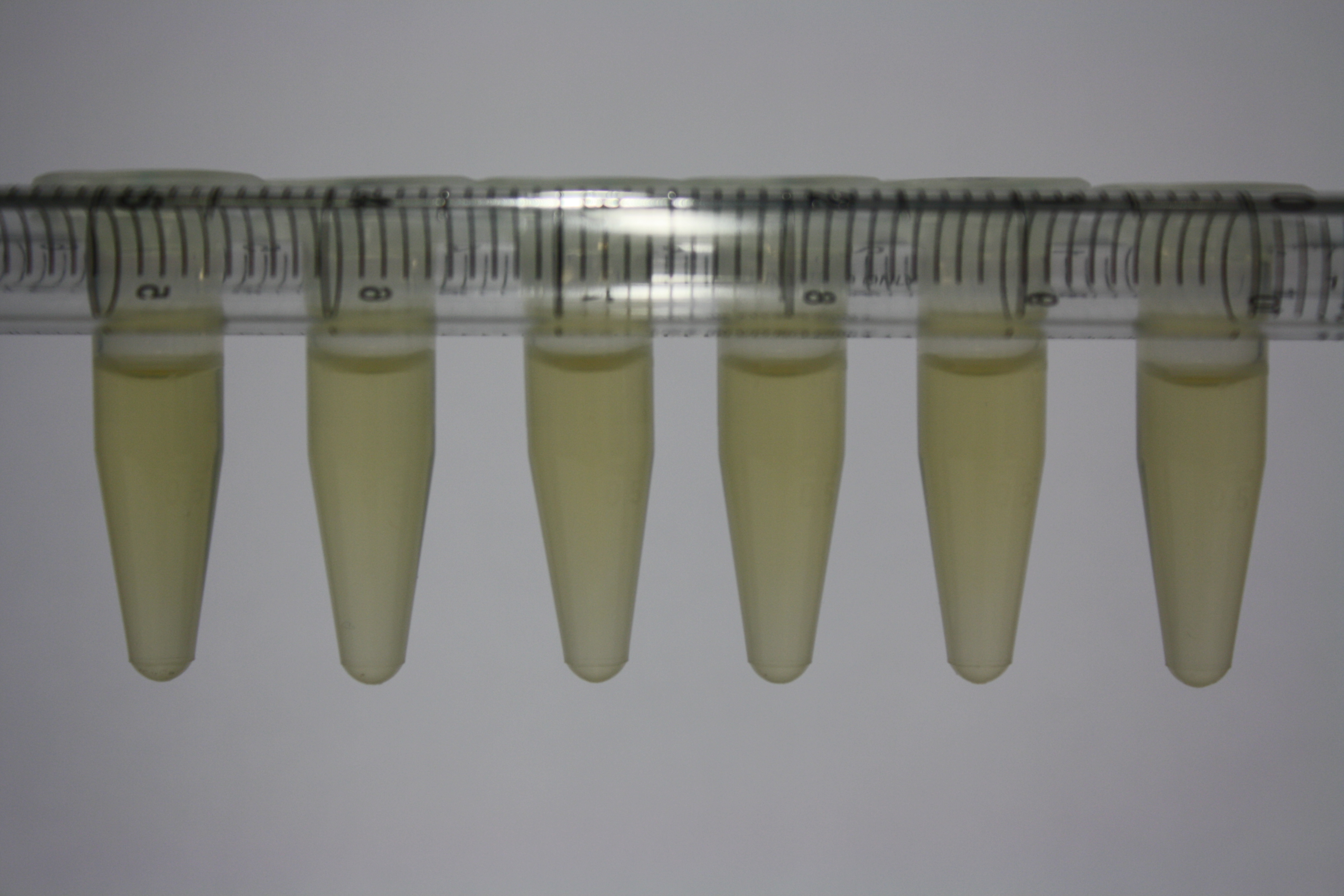
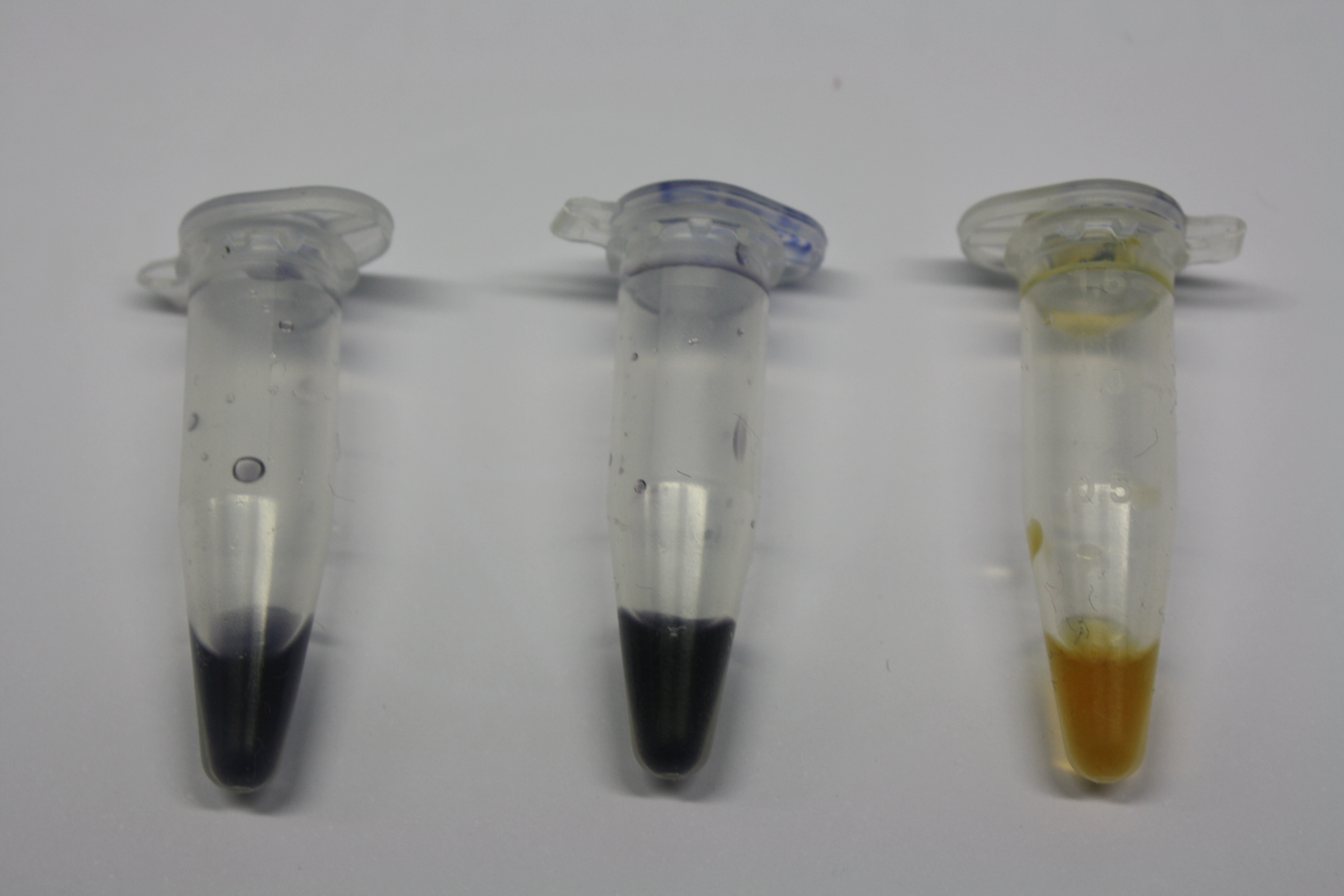
Different intermediate products were tested for their ability to precipitate gold. For this, 3 µl of AuCl3 (100g/l) were added to 500 µl solution. The outcome is presented in the following table.
| Sample | Precipitating Gold? | Colour of Precipitate | Time until Precipitation Started |
|---|---|---|---|
| AMC media | yes | grey | ~2 h |
| Supernatant of D. acidovorans SPH-1 culture | yes | black | 1:30 min |
| Supernatant after treatment with HP20 | yes | black | 1:40 min |
| HP20 after elution step | no | -- | -- |
| Methanol | no | -- | -- |
| Methanol used for elution | no | -- | -- |
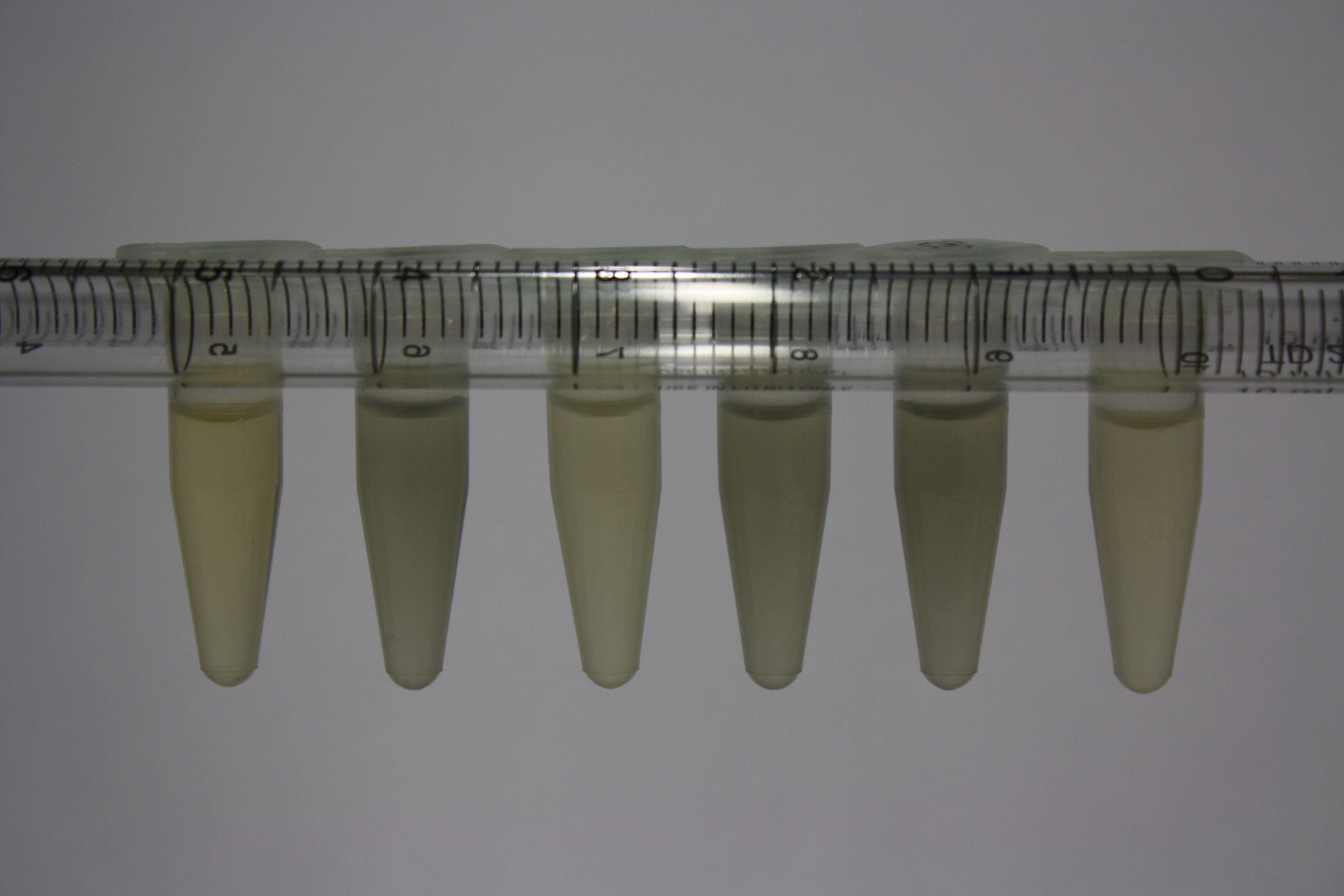
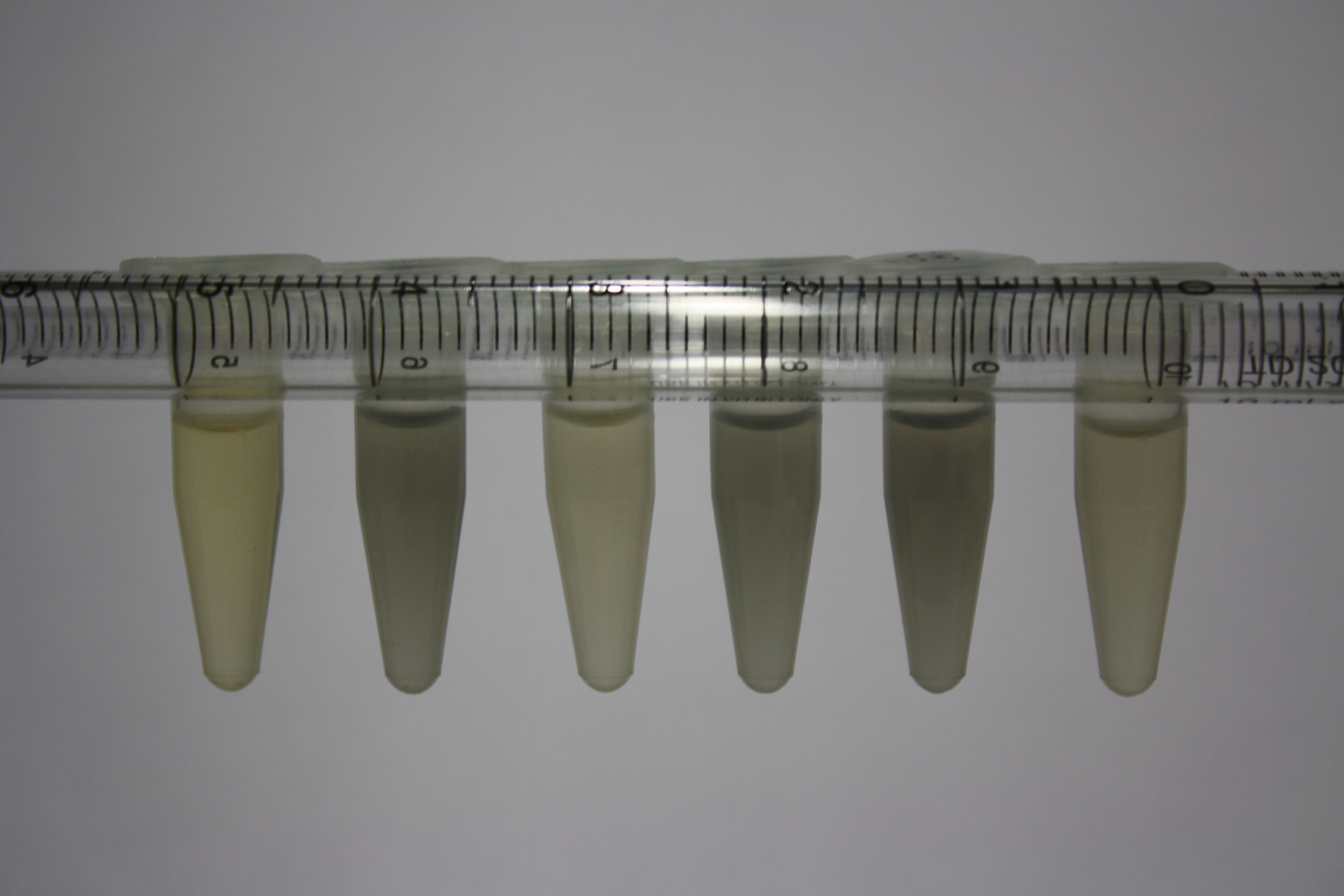
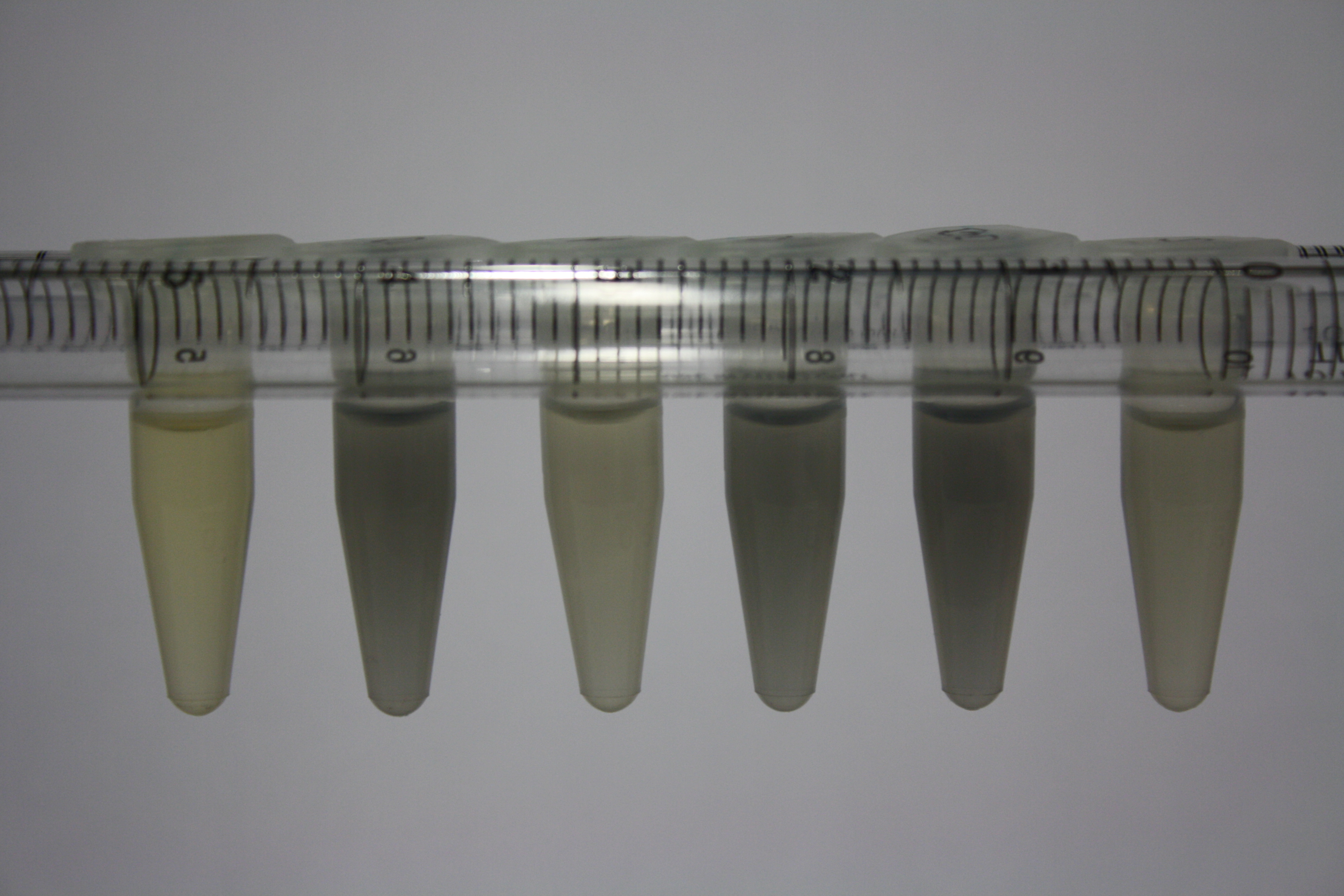
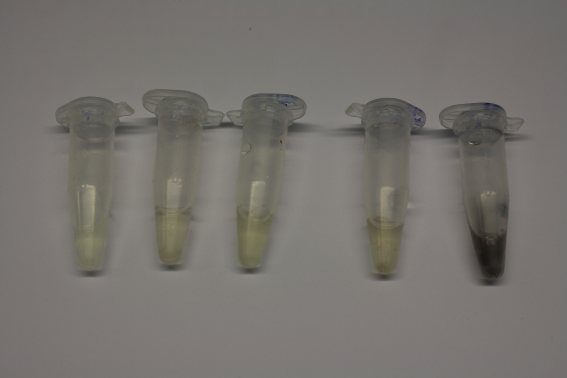
| Sample | Diluted in Water | Volume sample | Gold Solution 100g/l | Precipitating gold? | Colour of Precipitate | Time until Precipitation Started |
|---|---|---|---|---|---|---|
| ACM media | -- | 160 µl | 1 µl | yes | black | 1 h |
| Supernatant of D. acidovorans in ACM, filtered | -- | 160 µl | 1 µl | yes | black | 1 h |
| Purified Delftibactin after dilution in 50:50 Methanol, Water | -- | 160 µl | 1 µl | no | -- | -- |
| Water | -- | 250 µl | 1.5 µl | no | -- | -- |
| ACM media | 1:10 | 250 µl | 1.5 µl | no | -- | -- |
| Supernatant of D. acidovorans in ACM | 1:10 | 250 µl | 1.5 µl | no | -- | -- |
| Supernatant of D. acidovorans in ACM, filtered | 1:10 | 250 µl | 1.5 µl | no | -- | -- |
| Purified Delftibactin after dilution in 50:50 Methanol, H2O | 1:10 | 250 µl | 1.5 µl | yes | gray | over night |
Micro-TOF
The supernatant of a 24 h D. acidovorans SPH-1 culture was analysed before and after the purification procedure using a MICRO-TOF. As negative control unpurified ACM media was measured as well.
File:Heidelberg 20130911Malditof.pdf
Result
Delftibactin was clearly present in the purified supernatant of the D. acidovorans SPH-1 culture. In contrast it could not be detected in the ACM media or the supernatant which was not purified.
Triple Clone pIK8.6, DelH and DelH I 6B
Preparations
Electrocompetent E. coli DH10ß and BL21 DE3 were prepared as described.
Electroporation
We electroporated all three plasmids into E. coli DH10ß using pIK8.6. Although the DelH clone I 6B has a deletion in the ribosome binding site, we hope that we still get enough expression of DelH.
| Electroporation | Total amount of DNA [ng] | Ratio pIK2.6: DelH:DelRest | pIK8.6-1(12 Kb, 146 ng/µl) | DelH-I6B midi (23 Kb, 235.2 ng/µl) | DelRest (32 Kb, 2.4 µg/µl) |
|---|---|---|---|---|---|
| Triple into E. coli DH10ß | 774.2 | 1:2:3 | 1 µl of 1:10 (146 ng) | 1 µl (235.2 ng) | 1.5 µl of 1:10 (375 ng) |
Preparation of Cultures for Purification and MICRO-TOF
| Bacteria | Media | Antibiotics |
|---|---|---|
| -- | ACM | -- |
| E. coli DH10ß | ACM | -- |
| Delftia acidovorans SPH-1 | ACM | -- |
| E. coli DH10ß with plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 | ACM | Ampicillin, Chloramphenicol, Kanamycin |
- Inoculation of 10 ml of over night cultures of each sample
- Growth at 30°C (Delftia acidovorans) and 37°C (E. coli DH10ß and E. coli DH10ß + plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6)
- For each culture, 500 ml of ACM media with antibiotics was inoculated with the pre-cultures
- The cultures were grown for about 8 h.
ODs
- E. coli DH10ß = 1.2
- Delftia acidovorans SPH-1 = 0.4
- E. coli DH10ß + plasmids pHM04 (I6B), pFSN (D8w) & pIK8.6 = 0.8
Purification of Delftibactin
- Centrifugation of cultures at 3750 rpm for 30 min
- Retrieved supernatant
- Added 20 g/L HP-20
- Stirred at RT for 2 h
- Filtration with Buchner funnel to retain HP-20
- Washed with 400 ml dddH2O
- Elution with 200 ml Methanol
- Evaporated methanol with rotary evaporator
- Resuspended in 2 ml 50 methanol : 50 ddH2O
MICRO-TOF
File:Heidelberg Microtof0913.pdf
Result
Apparently the purification of delftibactin did not work, as even in the positive control (D. acidovorans SPH-1) no delftibactin was detected. As the cultures were harvested after only a short time of growth it is suspected that maybe the production of delftibactin had not started yet. The next time the cultures should grow for longer.
 "
"