Team:Heidelberg/Templates/Del week15 AF
From 2013.igem.org
Contents |
07-08-2013
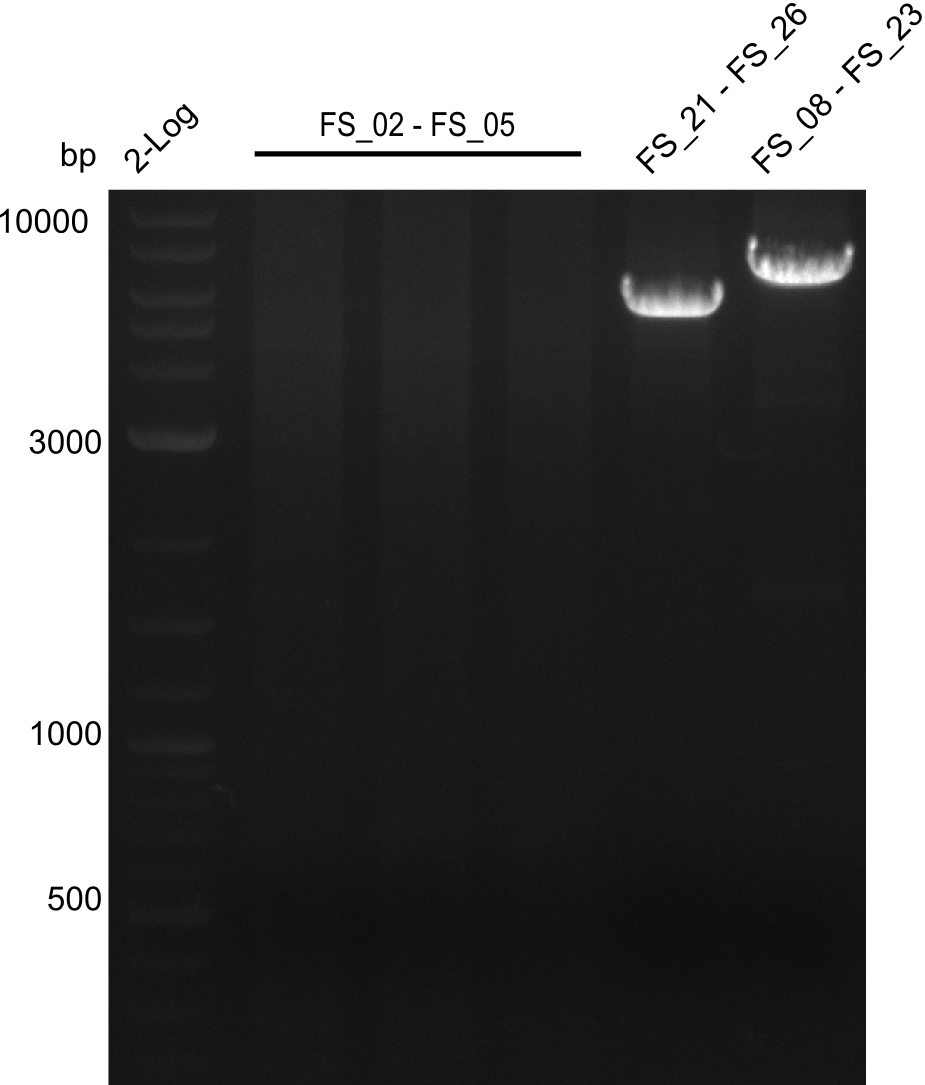
Amplification I from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
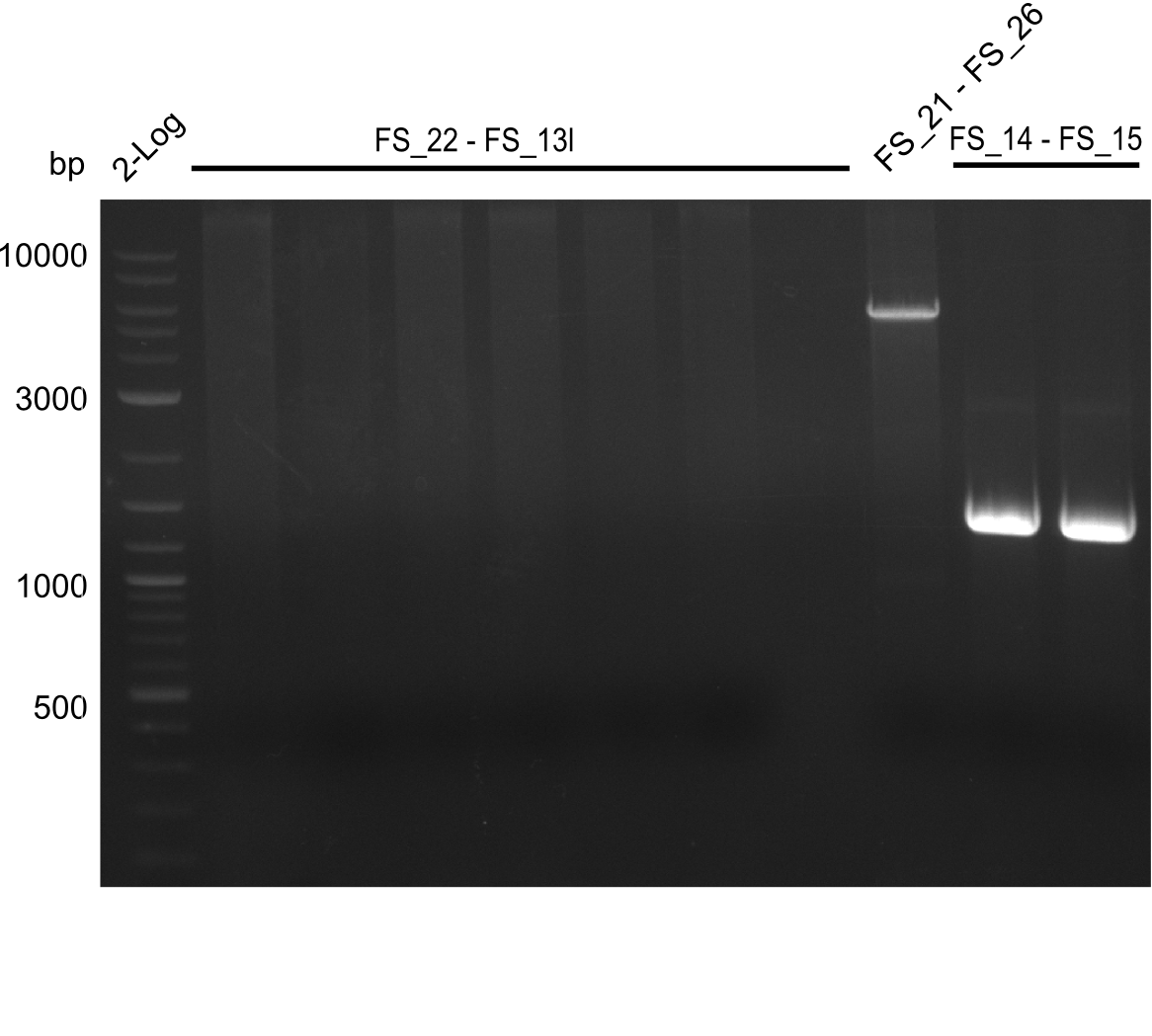
- Amplification of DelAF did not work
- Repeat amplification in case any errors were made
Amplification II from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
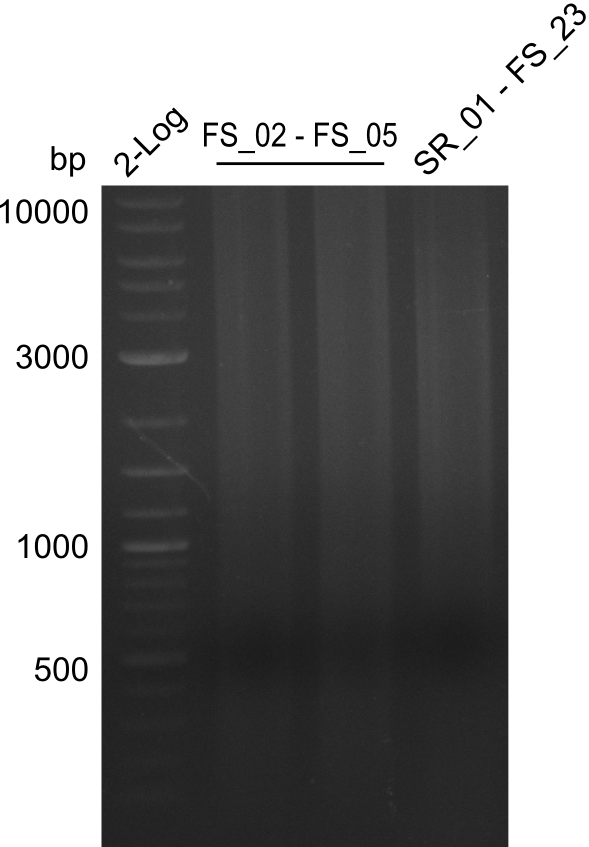
Results:
- Amplification of DelAF did not work as no product appeared, therefore conditions of PCR have to be optimized
- as extremely long amplicons in the past were usually easier to amplify from agar plate (colony PCR) the same conditions will be tried on the colony as template
08-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL |
|---|---|
| D. acidovorans SPH-1 (from agarplate) | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
- Amplification of DelAF did not work as no product appeared, therefore conditions of PCR have to be optimized
- PCR will be repeated with glycerol stock as template
09-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL 2nd PCR |
|---|---|
| D. acidovorans SPH-1 (glycerol stock) | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF did not work as no product appeared, therefore conditions of PCR have to be optimized
- PCR will be repeated with a higher annealing temperature as primers might not have bound due to secondary structes
- PCR will be repeated with lower annealing temperatue as primers might not have bound due to high temperatures
10-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF | |
|---|---|---|
| Template | D.acidovorans SPH-1 colony | |
| Primer fw | 2 µL FS_02 | 4 µL FS_02 |
| Primer rev | 2 µL FS_05 | 4 µL FS_05 |
| Phusion flash Ready Mix | 10 µL | |
| dd H2O | 1 µL | - |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 58 | 5 | |
| 72 | 3:15 | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF did not work at a temperature of 58°C constant
- as primers might not have been bound at this low temperatue but neither bound at a temperature of 68°C (touchdown or constant), PCR will be repeated at a temperature of 65°C as touchdown and at constant annealing
Amplification II + III from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF II + III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Template | D.acidovorans SPH-1 colony | |||||||||
| Primer fw | 4.5 µL FS_02 | |||||||||
| Primer rev | 4.5 µL FS_05 | |||||||||
| DMSO | 1 µL | |||||||||
| Phusion Ready Mix | 10 µL | |||||||||
- Conditions
| Biorad MyCycler | ||||||
|---|---|---|---|---|---|---|
| Cycles | temperature [°C] DelAF II | Time | Cycles | temperature [°C] DelAF III | Time | |
| 1 | 98 | 10 s | 1 | 98 | 10 s | |
| 30 | 98 | 1 s | 12 | 98 | 1 s | |
| 65 ↓ 0.5 | 5 s | |||||
| 65 | 5 s | 72 | 3:15 min | |||
| 18 | 98 | 1 s | ||||
| 72 | 3:15 min | 66 | 5 s | |||
| 72 | 3:15 min | |||||
| 1 | 72 | 10 min | 1 | 72 | 10 min | |
| 1 | 10 | inf | 1 | 10 | inf | |
Results:
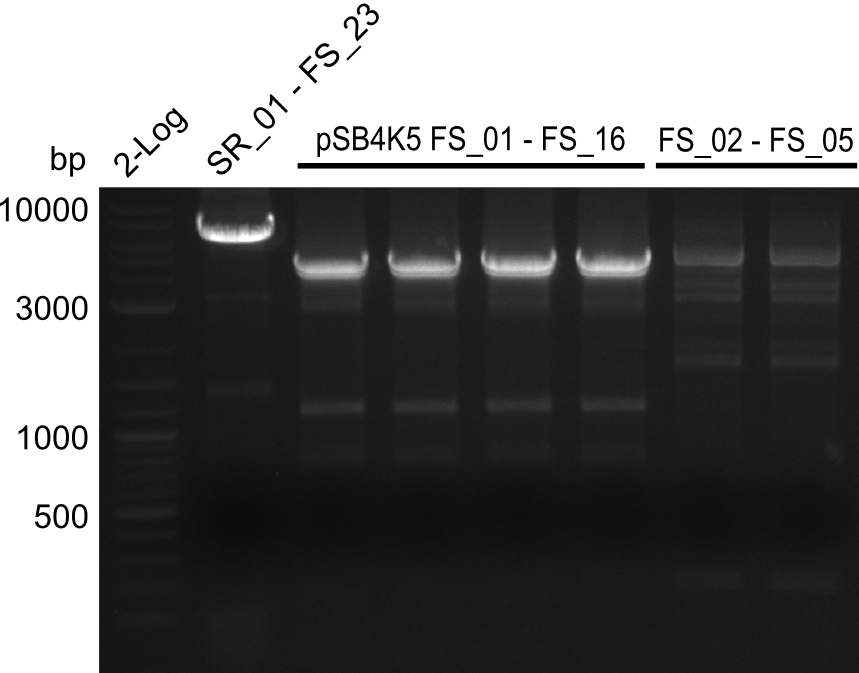
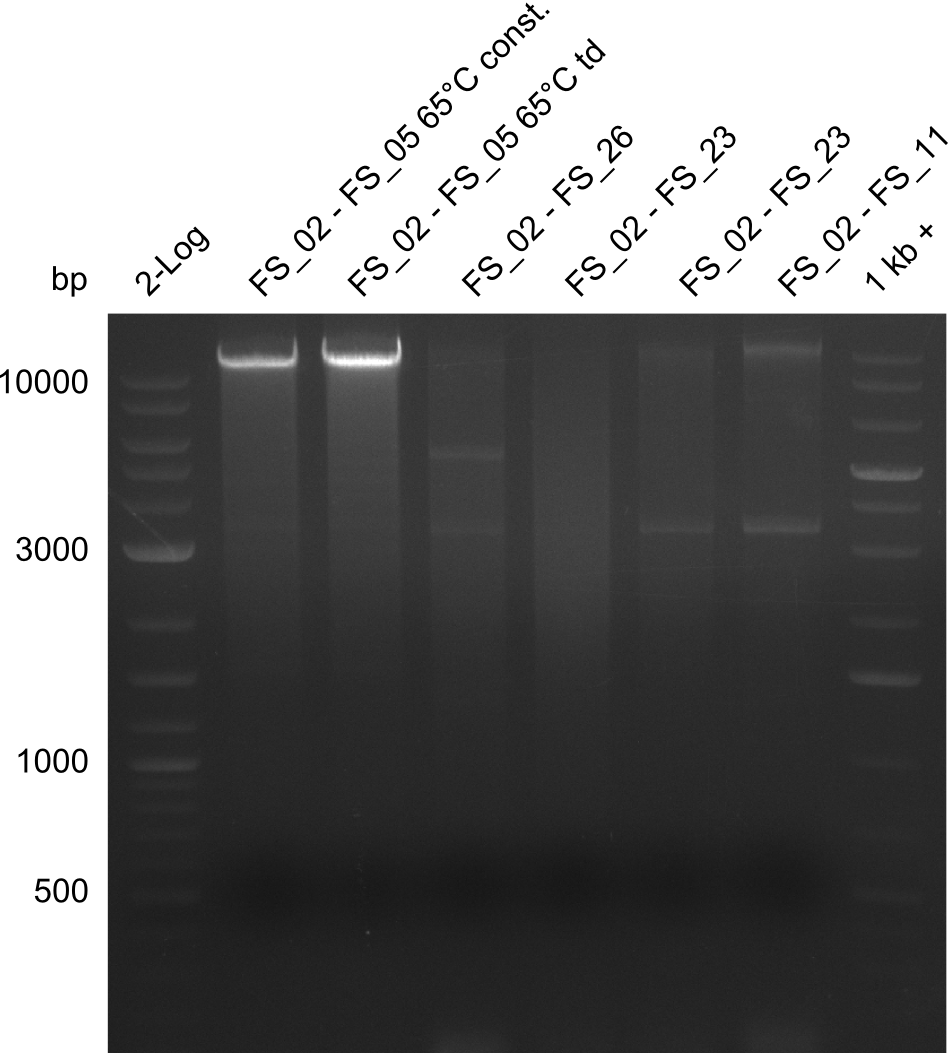
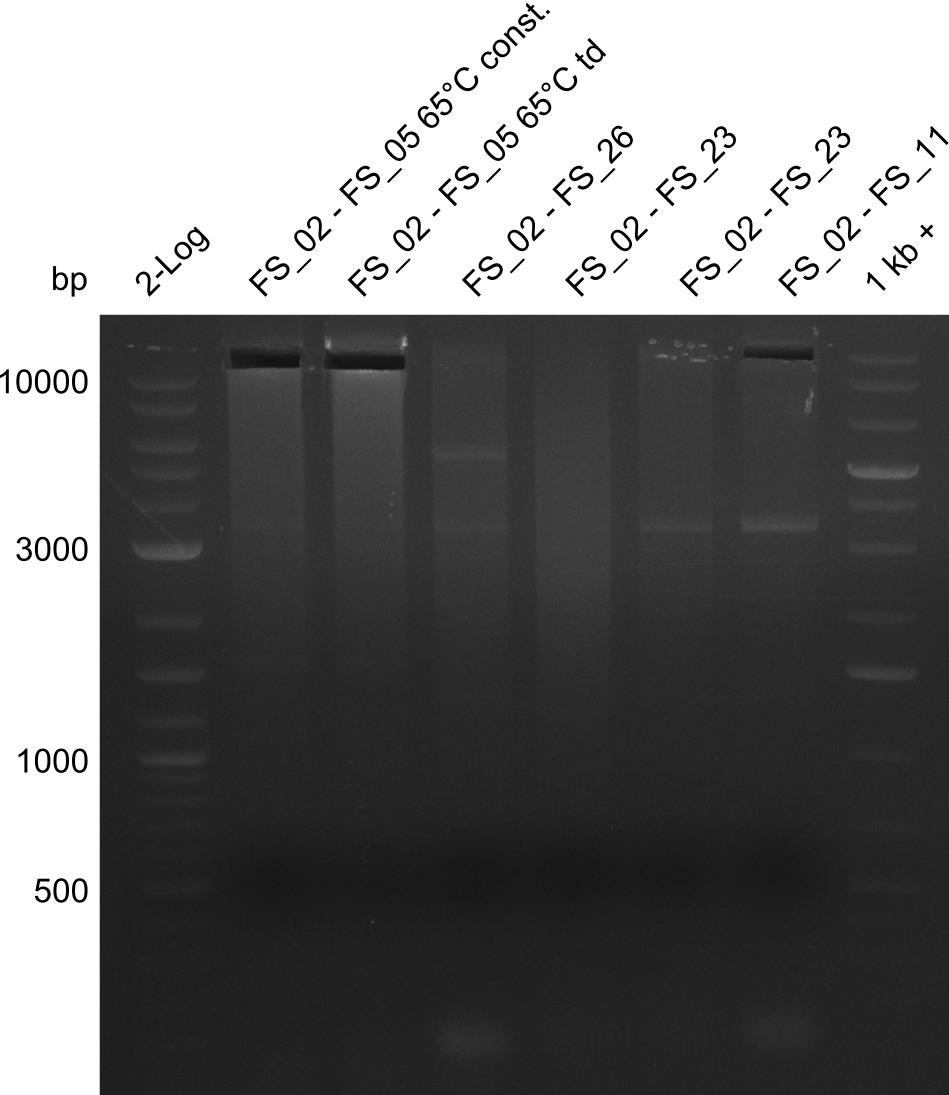
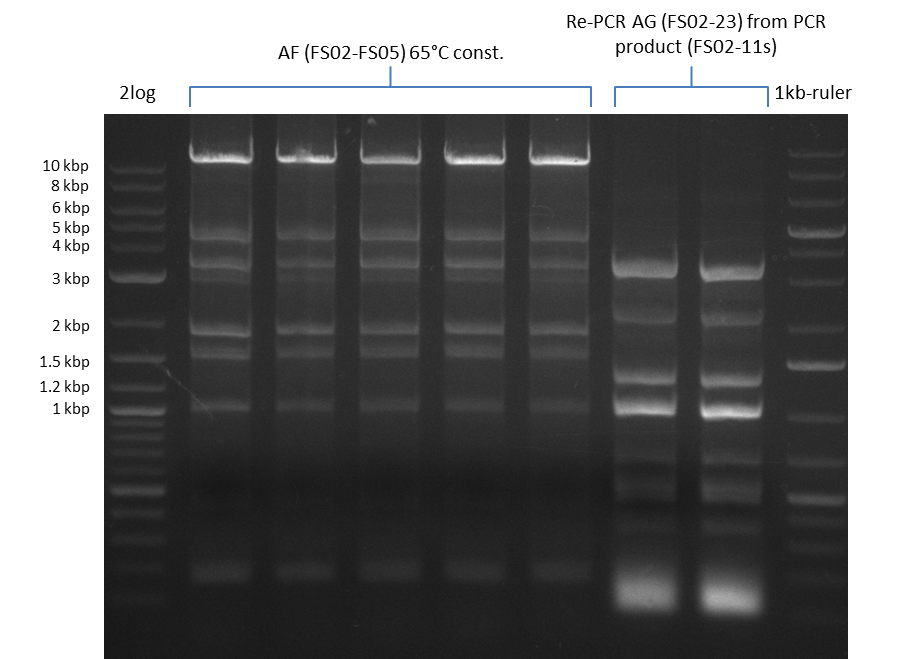
- Amplification of DelAF from the new strain finally worked, both conditions (65°C constant and 65°C touchdown) led to specific product but the touchdown PCR resulted in a smear
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- PCR will be repeated with a slightly lower temperature with constant annealing to increase yield but avoid the smear which appeared due to the low annealing temperatures used in the last cycles of the touchdown PCR.
11-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF |
|---|---|
| Template | D.acidovorans SPH-1 colony |
| Primer fw | 4.5 µL FS_02 |
| Primer rev | 4.5 µL FS_05 |
| Phusion Ready Mix | 10 µL |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 62 | 5 | |
| 72 | 3:15 | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
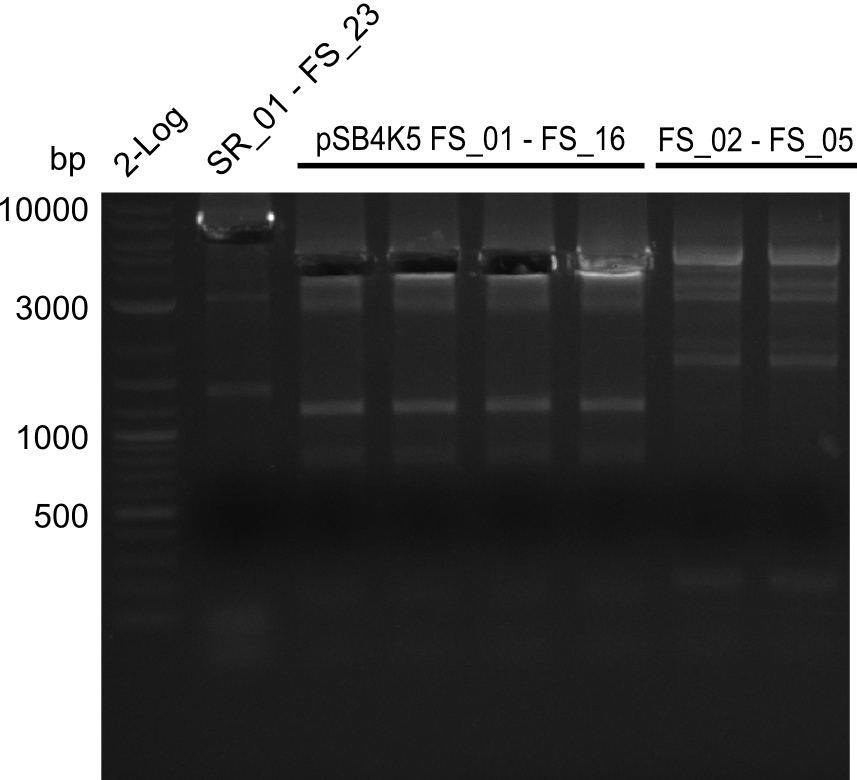
- Amplification of DelAF worked out though again a smear and several other bands appeared
- bands were cut out very precise to avoid carry over of any unwanted amplicon and DNA purified using QIAquick Gel Extraction Kit
- gradient PCR will be carried out to determine optimal annealing temperature of primers and thereby obtain product of higher specificity suitable for gibson assembly
 "
"