Laboratorio
From 2013.igem.org
Contents |
Parts
Notebook
Brainstorming
In the beginning, we decided to meet weekly to discuss ideas to be developed in iGEM competition. We scheduled a fixed day and time for such discussion and the presence of all members was considered mandatory, as we had in mind that a good project would require the involvement of everybody.
So, based on this perspective and considering that most of our colleagues have a knowhow applied to human health, we thought about tropical diseases, like dengue. We had imagined what we could do to precociously diagnose that disease as a measure to provide a fast and precise health care to patients positively diagnosed. Summarizing, we have firstly considered to develop a fast diagnostic tool for dengue, which everyone could use without restrictions, based on a GMO (Genetically Modified Organism).
Despite having made a big effort to implement it, many factors turned it an unviable project. First, we didn’t have means to deal with the vector, Aedes aegypti, and we couldn’t establish a viable way to use a GMO to our primary purposes. To try to solve these problems, we invited a researcher from Funed (Fundação Ezequiel Dias - Brazil), Alzira Batista Cecilio, to talk to us about the disease and the fast diagnostic test that she was developing in her studies. This gave us some possibilities, but all of them were too complex to be applied to iGEM in short time. We kept working on building new ideas to be implemented.
In order to perform a search, we divided our team in groups, which were encouraged to give new and viable ideas to be developed. One of them had a performable idea: check for biomarkers in order to precociously diagnose heart diseases, a priori based in choline detection. But, as this substance is released to blood flow in response to many disturbs, we thought that more biomarkers would be necessary in order to provide a reliable diagnosis. Occurred to us that it would be interesting to add a biomarker already validated and well described. We thought of using creatine-kinase MB (CK-MB), but it does not have an useable receptor or inducible promoter available, at least one that we could find, to be expressed on our chassis. Troponin was cogitated to be another of our relevant biomarkers, however, it has also shown to be unviable due to the absence of a receptor we could use and or a channel to transport it into the cell.
After an intense search, in the end we have agreed to use three biomarkers: Brain Natriuretic Peptide (BNP), Trimethylamine-N-Oxide (TMAO) and Ischemia Modified Albumin (IMA). BNP is a validated biomarker for Acute Coronary Syndrome (ACS) and it has a receptor that could be used to detect BNP, despite of huge size of its receptor (NPR-A) which have a transmembrane site. TMAO, which is not considered a validated biomarker, but it came out in our latest searches it could be used as a heart failure predictor, since this substance attacks the heart muscle tissue and provokes necrosis, the main factor of myocardial infarction. IMA is an indicative of any sort of ischemia and it was validated by FDA as a biomarker for ACS, although it is best used as a negative predictor than a positive one (meaning that its absence indicates that everything is probably fine, but its presence just hints that there might be something wrong).
Day by Day
January 2013
- Team UFMG was formed.
- We started having meetings every Tuesday’s afternoon to discuss our team project.
- Introductory presentations were given by members of the team to explain to the group the basic concepts involved in the iGEM competition, what Biobricks are, how computer science and biology can work together to create new living things, etc.
February and March 2013
- We discussed previous projects developed by iGEM teams and each team member was asked to bring new ideas for our project. After several presentations and discussions, we select the main theme of our project: cardiovascular diseases biomarkers.
April 2013
- On April 19th we presented our project to various Professors and graduate students from the Biochemistry and Immunology Department at UFMG. During these discussions we had the opportunity to present our initial ideas and discuss them with people that were not directly involved with the project. These discussions were very important since we received important feedback that helped us to improve designing our final proposal.([1])
- During all meetings the group had during this month, we discuss the literature about cardiac diseases biomarkers, in order to improve our project.
May 2013
- Our project has been improved and the definition of the biomarkers that we were going to assay became more and more clear.
- We started putting in place our ideas about the human practice components of our project.
- We published a text about our project in the SynbioBrasil’s blog, a blog created by the iGEM team from USP, see on: http://synbiobrasil.org/2013/05/28/minas-gerais-no-igem/.
June 2013
- The final design of our project was concluded and we received our iGEM’s biobricks kit.
July 2013
- We started our experiments by trying to grow the bacteria containing the plasmids, which was quite difficult because we had trouble with the cloramphenicol that we were using (it was expired and didn’t work well ). After solving that problem we were able to grow the cells and purify our plasmids.
- Biosafety Practices: we concluded an one-week course with Neuza Antunes about laboratory safety practices. ([2]).
- As part of the human Practices component of our project, we had a wonderful experience participating in the course UFMG & Escolas. This is a program that is being developed for many years in our University and has the goal of bringing high school students as well as school teachers to our campus to let them develop research projects according to their interests and curiosities. Teaching synthetic biology to those children and teenagers was quite enlightening. We used our Brickard Game to make it more attractive ([3]).
- We succeded in preparing our first construct after cloning of RCNA+YFP into PSB1A3.
August 2013
- We started fluorimetric assays with bacteria carrying the plasmid construct RCNA+YFP, to verify the effect of cobalt in the expression of YFP.
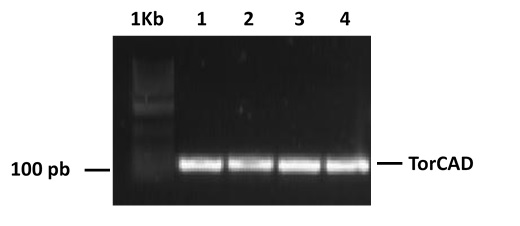
- We used the oligonucleotide that we had asked to be synthesized containing sequences of the TorCAD promoter and tried to clone this sequence into PSB1C3.
- We perform PCR and restriction enzyme digestion to confirm the identity of the constructs PSB1A3_RCNA+YFP and PSB1C3_TorCAD.
September 2013
- Additional fluorimetric assays were perfomed with bacteria transformed with PSB1A3_RCNA+YFP using different cobalt concentrations and in the presence of sera from normal mice or ischemic mice.
- We tried to clone the TorCAD promoter upstream RFP into the PSB1C3 plasmid.
- Our new biobricks were sent to iGEM Headquarters.
- We created “The E. coli Dilemma video” ([4]).
- On September 21th an interview with our team was published in one of the largest newspaper in the country, “Estado de Minas” – [5].
- 27th September: WIKI FREEZE!!!!
October 2013
- Regional Jamboree in Chile.
Protocols
1. Solid and liquid culture media 2xYT
For 1 liter of liquid medium:
- 16 g of tryptone
- 10 g of yeast extract
- 5 g NaCl
- Add ddH2O (di-deionized) to 1000 mL
For 1 liter of solid media
- Same compounds as liquid medium. - 3.95 grams of agar to 250 mL of liquid medium.
2. Chemically competent cell preparation
- In 5 mL of 2xYT media inoculate a clone of Escherichia coli and let it grow overnight, 37°C, 180 rpm.
- Inoculate 2 mL of E. coli culture in 200 mL of liquid culture medium in a recipient of 2 L. Grow it at 37°C, 250 rpm, until it reaches OD590 0.3 or 0.4.
- Divide aliquots of 50 mL in 4 conical tubes and let it in ice from 5 to 10 minutes.
- Centrifuge for 7 minutes, 4°C, 3000 rpm (~1600 x G).
- Purge the supernatant and resuspend each pellet obtained in a recipient with 5 mL of cold solution of CaCl2.
- Centrifuge the cells for 5 minutes, 4°C, 2500 rpm (~1333 x G). Repeat step 5 and let the cells in ice for 30 minutes.
- Repeat step 6, but using 1 mL of cold solution of CaCl2 to resuspend the cells. (Note: In this solution, cells can stay from 12 to 24 hours)
- Divide the cells in aliquots of 100 μL and freeze it at -80°C.
CaCl2 solution (100 mL):
- 60 mM CaCl2 - 0.882 g
- Glycerol 15% - 15 mL
- 10 mM PIPES - 0.3785 g (Note: Do not use PIPES free acid
- Sterilize (autoclave) and store at room temperature
3. CoCl2 solution preparation (250 mM)
- Weight 0.3246 g of CoCl2 (1 M = 129.84 g).
- Add 10 mL H2O to cobalt. Homogenize the mixture.
- Filter it using a 0.22 μm strainer.
4. DNA digestion
A) Single Reaction (10 μM)
DNA ---------------------------------------- 125 ng Buffer 10X* -------------------------------- 1 μL BSA 10 μg/mL ------------------------------- 1 μL Enzyme* ------------------------------------ 0.25 μL ddH2O (di-deionized) ----------------------- complet to 10 μL
*Enzyme and buffers used:
| Enzyme | Restriction site | Buffer |
| EcoRI | G↓AATC | ECOTango 1x- Fermentas |
| SpeI | A↓CTAGT | Tango 1x- Fermentas |
| XbaI | T↓CTAGA | Tango 1x- Fermentas |
| PstI | CTGCA↓G | OrangeTango 1x- Fermentas |
B) Cobalt promoter (RCNA) and reporter (YFP)
| DNA | Buffer10X | BSA 10X | ddH2O | Enzymes (each) | ||
| RCNA (BBa_K540001) | 5μL | 2μL | 2μL | 10μL | EcoRI + SpeI | 2μL |
| YFP(BBa_E0430) | 3.2μL | 2μL | 2μL | 11.3μL | XbaI + PstI | 0.5μL+1μL |
| Plasmid (PSB1A3) | 10μL | 2μL | 2μL | 5μL | EcoRI + PstI | 0.5μL |
C) TorCAD and Chloramphenicol plasmid resistance (PSB1C3)
| DNA | Buffer10X | BSA 10X | ddH2O | Enzymes (each) | ||
| PSB1C3 | 25ng/μL | 2μL | 2μL | 10μL | EcoRI + PstI | 2μL |
| TorCAD(BBa_K1086000) | 10ng/μL | 2μL | 2μL | 5μL | EcoRI + PstI | 0.5μL+1μL |
- 37ºC for 4 hours
D) TorCAD and PSB1C3-RFP
| DNA | Buffer10X | BSA 10X | ddH2O | Enzymes (each) | ||
| PSB1C3 | 25ng/μL | 2μL | 2μL | 10μL | EcoRI + XbaI | 2μL |
| TorCAD(BBa_K1086000) | 10ng/μL | 2μL | 2μL | 5μL | EcoRI + XbaI | 0.5μL+1μL |
- 37ºC for 4 hours
5. Ligation
A) RCNA-YFP-PSB1A3 (10μL)
Plasmid (PSB1A3) --------------------- 2 μL RCNA (BBa_K540001) ------------------- 2.5 μL YFP (BBa_E0430) ---------------------- 2.5 μL Buffer 10X --------------------------- 1 μL T4 DNA Ligase ------------------------ 1 μL ddH2O (di-deionized) ----------------- 1 μL
- Incubate it overnight at 4ºC.
B) PSB1C3 and TorCAD (10μL)
Plasmid (PSB1C3) --------------------- 2 μL TorCAD (BBa_K1086000) ---------------- 2.5 μL Buffer 10X --------------------------- 1 μL T4 DNA Ligase ------------------------ 1 μL ddH2O (di-deionized) ----------------- 3.5 μL
- Incubate it overnight at 4ºC.
C) PSB1C3-RFP and TorCAD (10μL)
Plasmid (PSB1C3-RFP) ----------------- 2 μL TorCAD (BBa_K1086000) ---------------- 2.5 μL Buffer 10X --------------------------- 1 μL T4 DNA Ligase ------------------------ 1 μL ddH2O (di-deionized) ----------------- 3.5 μL
- Incubate it overnight at 4ºC.
6. Transformation Protocol (as suggested by iGEM-registry)
- Start thawing the chemically competent cells on ice.
- Add 1 - 2 μL of DNA to the 2 mL tube. Pipet up and down a few time, gently. Make sure to keep the competent cells on ice.
- Close the tubes and incubate the cells on ice for 30 minutes.
- Add cells tubes by immersion in a preheated water bath at 42 °C for 60 seconds.
- Incubate the cells on ice for 5 minutes.
- Add 400 μL of 2xYT media (make sure that the broth does not contains antibiotics and is not contaminated) to each transformation.
- Incubate the cells at 37°C for 1 hour while the tubes are rotating or shaking.
Important: 2 hours recovery time helps in transformation efficiency, especially for plasmid backbones with antibiotic resistance other than ampicillin.
- Label two petri dishes with 2xYT agar (AMP or CHL). Plate 20 μL and 200 μL of the transformation onto the dishes, and spread. This helps you ensure that you will be able pick out a single colony.
- Incubate the plates at 37°C for 12-14 hours, making sure the agar side of the plate is up. If incubated for too long the antibiotics start break down and untransformed cells will begin to grow, because the resistance enzyme will be excreted by the bacteria inactivating the antibiotic outside of it.
- Pick a single colony, make a glycerol stock, grow up a cell culture and miniprep.
7. Miniprep
- We used Promega (Wizard® Plus SV Minipreps DNA Purification Systems) and Invitrogen (PureLink™ Quick Plasmid Miniprep Kit) kits. We followed manufacturer’s indications.
8. PCR Protocol
Compound ------------------------ Volume ddH2O --------------------------- 8.0 μL Buffer IB 10x ------------------- 1.5 μL dNTP’s 2.5 mM ------------------- 1.5 μL Primer VF2 10 uM ---------------- 0.4 μL Primer VR 10 uM ----------------- 0.4 μL Taq 5u/ μL ---------------------- 0.2 μL DNA ----------------------------- 3.0 μL Final volume -------------------- 15 μL
Amplifications were performed in M.J. Research PTC-100 (GMI Inc.) thermocyclers. The amplification program was as follows:
- 10 minutes of initial denaturation at 94ºC.
- 30 cycles of denaturation (94ºC for 1 minute), annealing (55ºC for 1 minute) and extension (72ºC for 1 minute).
- 10 minutes of final extension at 72ºC.
- PCR products were analysed in 1% agarose gels, using 1Kb DNA Ladder (Invitrogen) as molecular weight ladder. Gels were stained with Sybr Safe (Life Technologies).
9. Fluorimetric assay for RCNA-YFP activation (Protocol # 1):
- Measure OD600 of cultures to be assessed.
- Dilute cultures to OD600 = 0.02, in a volume of 3 mL.
- Prepare a solution of CoCl2 1 mM, using 6 μL of 250 mM solution (see protocol 3) and 1494 μL of 2xYT media.
- Make other 6 cobalt solutions using the mixture on step (3).
50 uM -> 50 μL of 1 mM solution + 950 μL of media 2xYT. 100 uM -> 100 μL of 1 mM solution + 900 μL of 2xYT media. 150 uM -> 150 μL of 1 mM solution + 850 μL of 2xYT media. 200 uM -> 200 μL of 1 mM solution + 800 μL of 2xYT media. 250 uM -> 250 μL of 1 mM solution + 750 μL of 2xYT media. 300 uM -> 300 μL of 1 mM solution + 700 μL of 2xYT media.
- In a 96 well plate, add 100 μL of the cultures from step (2) in each well, in triplicate.
- Make the blank: add 100 μL of media 2xYT in another wells, to discard the noise from media and cobalt.
- Add 100 μL of cobalt solutions from step (4) to cultures and to the blank:
- To a final concentration of 25 μM, use 100 μL of the 50 μM cobalt solution;
- To a final concentration of 50 μM, use 100 μL of the 100 μM cobalt solution;
- To a final concentration of 75 μM, use 100 μL of the 150 μM cobalt solution;
- And so on….
- Seal the plate.
- Read fluorescence: 514 nm (excitation) and 527 nm (emission), every 15 minutes, for 16 hours.
Note: It is not recommended to read absorbance using black plate, as it causes interference on reading, despite it being usually utlized for fluorimetric assay.
10. Fluorimetric assay for RCNA-YFP activation (Protocol # 2):
- Measure OD600 of bacterial cultures.
- Dilute cultures to OD600 = 0.1.
- Let bacteria grow until OD600 = 0.5.
- Proceed as protocol #9, from steps 3 to 8.
- Read absorbance at 600 nm and fluorescence at 514 nm (excitation) and 527 nm (emission), every hour, until 4 hours after the first read. Let bacteria grow for more 4 hours and then read absorbance and fluorescence every 2 hours, for 16 hours.
11. Hindlimb ischemia model
- The blood was donated by Angiogenesis Laboratory from Biological Science Institute in UFMG where it was collected from brachial plexus of the mice according to ethic committee approval from CETEA-UFMG, licence 253/08.
- The Serum obtained was centrifuged by 10 minutes 4000 rpm in room temperature.
References
- Madeddu P; Emanueli C; Spillmann F; Meloni M; Bouby N; Richer C; Alhenc-Gelas F; Van Weel V; Eefting D; Quax PH; Hu Y; Xu Q; Hemdahl AL; van Golde J; Huijberts M; de Lussanet Q; Struijker Boudier H; Couffinhal T; Duplaa C; Chimenti S; Staszewsky L; Latini R; Baumans V; Levy BI. Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems.Vascul Pharmacol. 2006;45:281-301.
- Madeddu P; Kraenkel N; Barcelos LS; Siragusa M; Campagnolo P; Oikawa A; Caporali A; Herman A; Azzolino O; Barberis L; Perino A; Damilano F; Emanueli C; Hirsch E. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol. 2008 (1):68-76.
Image References
- Niiyama H, Huang NF, Rollins MD, Cooke JP. Murine model of hindlimb ischemia. J Vis Exp. (JoVE) 2009 Jan 21;(23). doi:pii: 1035. 10.3791/1035.
- Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hindlimb ischemia. Nat Protoc. 2009;4(12):1737-46. doi: 10.1038/nprot.2009.185.
12. IMA binding assay: test of bacteria with RCNA-YFP using mice serum
- Follow the steps 1 to 3 from Protocol #10
- Prepare a cobalt (CoCl2) solution in culture media at 100 mM. This is the blank solution. Distribute it in 7 wells.
- Distribute 100 μL of media with bacteria in 12 wells. Add 100 μL of mice sera (3 serum for control and 3 serum with ischemia), in duplicates.
- For the positive control, add media with cobalt instead of the serum.
- Do the same as step #5 but adding the ischemic mice serum.
- Read fluorescence at 514 nm (excitation) and 527 nm (emission) every hour during 16 hours.
13. BSA-Cobalt binding assay
- Follow the steps 1 to 3 from Protocol #10
- Prepare a solution of cobalt (CoCl2) in culture media at 100 mM. Use this is as blank solution
- Prepare the solution of BSA at 66 mg/ml in media with cobalt. Make a serial dilution to obtain different BSA concentrations: 66 mg/ml, 33 mg/ml, 16.5 mg/ml, 8.25 mg/ml, 4.125 mg/ml, 2.063, mg/ml 1.032 mg/ml, 0.516 mg/ml, 0.258 mg/ml, 0.129 mg/ml, 0.065 mg/ml, 0.033 mg/ml, 0.017 mg/ml, 0.009 mg/ml, 0.005 mg/ml, 0.003 mg/ml
- Make triplicates for each BSA solution earlier made in step 3.
- In each well, add 100 uL of bacteria culture obtained in step 1.
- Seal the plate.
- Read fluorescence at 514 nm (excitation) and 527 nm (emission) every hour during 16 hours.
14. Fluorimetric assay for TorR+RFP activation
- Proceed as steps 1 to 3 from protocol #10.
- Prepare a solution of TMAO in media with a concentration of 100 mM. Make a serial dilution, to obtain different TMAO concentrations: 100 mM, 10 mM, 1 mM, 100 μM, 10 μM, 1 μM
- Prepare the blank solution with culture media and TMAO. And pipette it on 7 wells.
- Pipette the TMAO solutions with different concentrations, making triplicates for each one.
- Add 100 μL of bacteria culture obtained in step 1 and add it in each well from step 4.
- Seal the plate. Read fluorescence at 610 nm (excitation) 587 nm (emission)
Sequences
Sequences we send to synthesize
PromTorCAD (125 bp) :
5'-CGAACGAATTCGCGGCCGCTTCTAGAGATTCTGTTCATATCTGTTCATATTCCGTTCATCCTGACCAGTGCCGCTGTTCATATTTGCTCATTAAGATCGCTT CATACTAGTAGCGGCCGCTGCAG - 3'
PromPDE5_OM (180 bp):
5'-GAATTCGCGGCCGCTTCTAGGGCCGCGCGGCCTCCTTCCAGCCGCCGCCACTTGGCTTCCGGAGAGCTCGCCGGGCGCTGCCGCCGCCGCCGCCGCCGCCTC CTGGGAACCAGGGGACTGAAGAGCCTGCGAGAGCGGAACACTGCCGGACCCCGGGTGTACTAGTAGCGGCCGCTGCAG - 3'
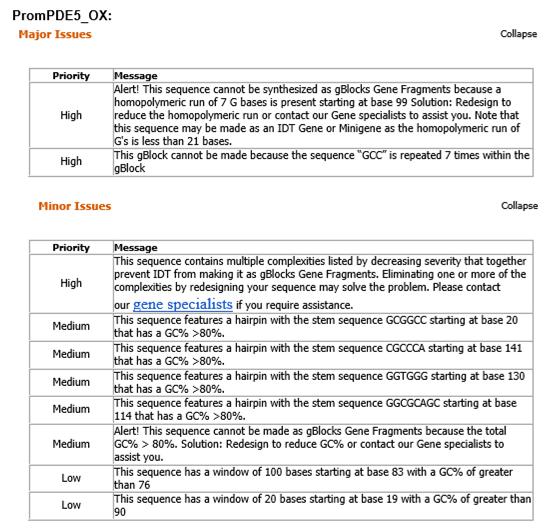
PromPDE5_OX (275 bp):
5'-GAATTCGCGGCCGCTTCTAGGCCGCCGCCGCCGCCGCCGCCTCCTGGGAACCAGGGGACTGAAGAGCCTGCGAGAGCGGAACACTGCCGGACCCCGGGTGGG GGGGCGCAGCAGCTGCGCCTGGCCCCGCCCACCACACCTGGGCGCCCGTAGAACCGCGCGGGGCGGGGCGGGGCAGGAGGCTGGCCTGGCGCTCCGGCCGCTTTG TCGAAAGCCGGCCCGACTGGAGCAGGACGAAGGGGGAGGGTCTCGAGTACTAGTAGCGGCCGCTGCAG - 3'
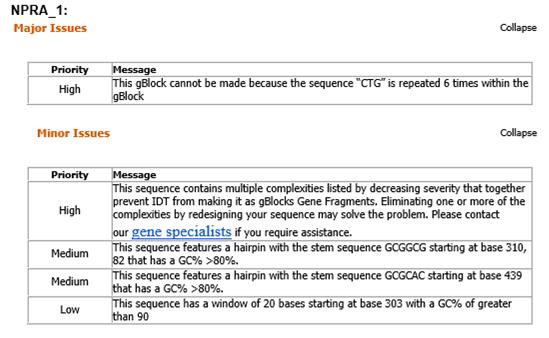
NPRA_1 (473 bp):
5’-GAATTCGCGGCCGCTTCTAGATGCCGGGTCCGCGTCGTCCGGCGGGTTCTCGTCTGCGTCTGCTGCTGCTGCTGCTGCTGCCGCCGCTGCTGCTGCTGCTGC GTGGTTCTCACGCGGGTAACCTGACCGTTGCGGTTGTTCTGCCGCTGGCGAACACCTCTTACCCGTGGTCTTGGGCGCGTGTTGGTCCGGCGGTTGAACTGGCGC TGGCGCAGGTTAAAGCGCGTCCGGACCTGCTGCCGGGTTGGACCGTTCGTACCGTTCTGGGTTCTTCTGAAAACGCGCTGGGTGTTTGCTCTGACACCGCGGCGC CGCTGGCGGCGGTTGACCTGAAATGGGAACACAACCCGGCGGTTTTCCTGGGTCCGGGTTGCGTTTACGCGGCGGCGCCGGTTGGTCGTTTCACCGCGCACTGGC GTGTTCCGCTGCTGACCGCGGGTGCGCCGGCGCTGGGTTTCGGTGTTAAAGACGAA-3’
NPRA_2 (497 bp):
5’-CCGGCGCTGGGTTTCGGTGTTAAAGACGAATACGCGCTGACCACCCGTGCGGGTCCGTCTTACGCGAAACTGGGTGACTTCGTTGCGGCGCTGCACCGTCGT CTGGGTTGGGAACGTCAGGCGCTGATGCTGTACGCGTACCGTCCGGGTGACGAAGAACACTGCTTCTTCCTGGTTGAAGGTCTGTTCATGCGTGTTCGTGACCGT CTGAACATCACCGTTGACCACCTGGAATTTGCGGAAGACGACCTGTCTCACTACACCCGTCTGCTGCGTACCATGCCGCGTAAAGGTCGTGTTATCTACATCTGC TCTTCTCCGGACGCGTTCCGTACCCTGATGCTGCTGGCGCTGGAAGCGGGTCTGTGCGGTGAAGACTACGTTTTCTTCCACCTGGACATCTTCGGTCAGTCTCTG CAAGGTGGTCAGGGTCCGGCGCCGCGTCGTCCGTGGGAACGTGGTGACGGTCAGGACGTTTCTGCGCGTCAGGCGTTCCA – 3’
NPRA_3 (497 bp):
5’-TCAGGACGTTTCTGCGCGTCAGGCGTTCCAGGCGGCGAAAATCATCACCTACAAAGACCCGGACAACCCGGAATACCTGGAATTTCTGAAACAGCTGAAAC ACCTGGCGTACGAACAGTTCAACTTCACCATGGAAGACGGTCTGGTTAACACCATCCCGGCGTCTTTCCACGACGGTCTGCTGCTGTACATCCAGGCGGTTACC GAAACCCTGGCGCACGGTGGTACCGTTACCGACGGTGAAAACATCACCCAGCGTATGTGGAACCGTTCTTTCCAGGGTGTTACCGGTTACCTGAAAATCGACTC TTCTGGTGACCGTGAAACCGACTTCTCTCTGTGGGACATGGACCCGGAAAACGGTGCGTTCCGTGTTGTTCTGAACTACAACGGTACCTCTCAGGAACTGGTTG CGGTTTCTGGTCGTAAACTGAACTGGCCGCTGGGTTACCCGCCGCCGGACATCCCGAAATGCGGTTTCGACAACGAAGACCCGG – 3’
NPRA_4 (495 bp):
5’-CGAAATGCGGTTTCGACAACGAAGACCCGGCGTGCAACCAGGACCACCTGTCTACCCTGGAAGTTCTGGCGCTGGTTGGTTCTCTGTCTCTGCTGGGTATC CTGATCGTTTCTTTCTTCATCTACCGTAAAATGCAGCTGGAAAAAGAACTGGCGTCTGAACTGTGGCGTGTTCGTTGGGAAGACGTTGAACCGTCTTCTCTGGA ACGTCACCTGCGTTCTGCGGGTTCTCGTCTGACCCTGTCTGGTCGTGGTTCTAACTACGGTTCTCTGCTGACCACCGAAGGTCAGTTCCAGGTTTTCGCGAAAA CCGCGTACTACAAAGGTAACCTGGTTGCGGTTAAACGTGTTAACCGTAAACGTATCGAACTGACCCGTAAAGTTCTGTTCGAACTGAAACACATGCGTGACGTT CAGAACGAACACCTGACCCGTTTCGTTGGTGCGTGCACCGACCCGCCGAACATCTGCATCCTGACCGAATACTGCCCGCGTG – 3’
NPRA_5 (491 bp):
5’-TCTGCATCCTGACCGAATACTGCCCGCGTGGTTCTCTGCAAGACATCCTGGAAAACGAATCTATCACCCTGGACTGGATGTTCCGTTACTCTCTGACCAAC GACATCGTTAAAGGTATGCTGTTCCTGCACAACGGTGCGATCTGCTCTCACGGTAACCTGAAATCTTCTAACTGCGTTGTTGACGGTCGTTTCGTTCTGAAAAT CACCGACTACGGTCTGGAATCTTTCCGTGACCTGGACCCGGAACAGGGTCACACCGTTTACGCGAAAAAACTGTGGACCGCGCCGGAACTGCTGCGTATGGCGT CTCCGCCGGTTCGTGGTTCTCAGGCGGGTGACGTTTACTCTTTCGGTATCATCCTGCAAGAAATCGCGCTGCGTTCTGGTGTTTTCCACGTTGAAGGTCTGGAC CTGTCTCCGAAAGAAATCATCGAACGTGTTACCCGTGGTGAACAGCCGCCGTTCCGTCCGTCTCTGGCGCTGCAATCT – 3’
NPRA_6 (495 bp):
5’-CCGTTCCGTCCGTCTCTGGCGCTGCAATCTCACCTGGAAGAACTGGGTCTGCTGATGCAGCGTTGCTGGGCGGAAGACCCGCAGGAACGTCCGCCGTTCCA GCAGATCCGTCTGACCCTGCGTAAATTCAACCGTGAAAACTCTTCTAACATCCTGGACAACCTGCTGTCTCGTATGGAACAGTACGCGAACAACCTGGAAGAAC TGGTTGAAGAACGTACCCAGGCGTACCTGGAAGAAAAACGTAAAGCGGAAGCGCTGCTGTACCAGATCCTGCCGCACTCTGTTGCGGAACAGCTGAAACGTGGT GAAACCGTTCAGGCGGAAGCGTTCGACTCTGTTACCATCTACTTCTCTGACATCGTTGGTTTCACCGCGCTGTCTGCGGAATCTACCCCGATGCAGGTTGTTAC CCTGCTGAACGACCTGTACACCTGCTTCGACGCGGTTATCGACAACTTCGACGTTTACAAAGTTGAAACCATCGGTGACGCG – 3’
NPRA_7 (459 bp):
5’-GTTTACAAAGTTGAAACCATCGGTGACGCGTACATGGTTGTTTCTGGTCTGCCGGTTCGTAACGGTCGTCTGCACGCGTGCGAAGTTGCGCGTATGGCGCT GGCGCTGCTGGACGCGGTTCGTTCTTTCCGTATCCGTCACCGTCCGCAGGAACAGCTGCGTCTGCGTATCGGTATCCACACCGGTCCGGTTTGCGCGGGTGTTG TTGGTCTGAAAATGCCGCGTTACTGCCTGTTCGGTGACACCGTTAACACCGCGTCTCGTATGGAATCTAACGGTGAAGCGCTGAAAATCCACCTGTCTTCTGAA ACCAAAGCGGTTCTGGAAGAATTTGGTGGTTTCGAACTGGAACTGCGTGGTGACGTTGAAATGAAAGGTAAAGGTAAAGTTCGTACCTACTGGCTGCTGGGTGA ACGTGGTTCTTCTACCCGTGGTTAATACTAGTAGCGGCCGCTGCAG – 3’
What are these sequences?
PromTorCAD:
- Promoter indirectly sensitive to TMAO.
PromPDE5_OM:
- Promoter of PDE5 (phosphodiesterase 5), sensitive to cGMP. It was going to be used to indirectly detect BNP.
PromPDE5_OX:
- Promoter of PDE5 (phosphodiesterase 5), sensitive to cGMP. It was going to be used to indirectly detect BNP.
NPRA_1 to 7:
- Human receptor for BNP. The sequence was divided into 7 parts, called gBlocks, because it was too large to be synthesized as one sequence. The 7 parts were going to be united by using the kit Gibson Assembly Master Mix (NEB catalog #E2611), from New England Biolabs.
Huston, we have a problem!!!
- When the sales representant from Síntese Biotecnologia, Juliana Pimenta, tried to make the request of our sequences, IDT software refused 3 of them:
Sad Decision...
- Since 2 of the refused sequences were of the promoters that we were going to use to detect BNP, and we couldn’t change these sequences, as they are not translated (no codon usage), we decided to give up on BNP. If we had more time, we would have looked for other promoters that could be used.
- Hence, only the sequence PromTorCAD was synthesized.
Primers
- Forward primer for sequencing/amplifying BioBrick parts (VF2):
VF2: 5’- TGCCACCTGACGTCTAAGAA – 3’ (20 pb)
- Reverse primer for sequencing/amplifying BioBrick parts (VR):
VR: 5’- ATTACCGCCTTTGAGTGAGC – 3’ (20 pb)
- Forward and reverse primers for sequencing/amplifying RCNA promoter:
RCNFW: 5’- ATG AAT CCA GCA CCT TCA GAA C -3’ (22 pb)
RCNRV: 5’- CTT AGT ATT AAT TCG GCA ATC TGA TTC TAC TCC -3’ (33 pb)
- Forward and reverse primers for sequencing/amplifying TMAO promoter:
TORRFW: 5’- ATT CTG TTC ATA TCT GTT CAT ATT CCG TTC ATC CTG -3’ (36 pb)
TORRRV: 5’- TGA AGC GAT CTT AAT GAG CAA ATA TGA ACA GC -3’ (32 pb)
- Forward and reverse primers for sequencing/amplifying YFP/CFP/RFP:
FLUOFW: 5’- AAA GAG GAG AAA TAC TAG ATG GTG AGC AAG -3’ (30 pb)
FLUORV: 5’- TAT AAA CGC AGA AAG GCC CAC -3’ (21 pb)
Safety
Course
Biosafety Course
Before performing experiments, we were invited to know more about the lab routine and the procedures required to cope with organisms genetically modified and compounds usually required to perform experiments as well. Everyone interested in performing experiments during the competition was invited to a course to receive instructions and to learn more about the biosafety and its implications on lab working.
The central idea of this course was discuss about what is biosafety, why this is important and what is its implications on lab working. Under this perspective we started reading several texts to increase our knowledge about the subjects listed below :
The book Manual de biossegurança[1] (Biosafety Manual), a collection of texts written focused to teach the reader the most important procedures for control, handling and discard of biological products, for avoiding experiment contamination and the vigent law (at least the main) related to manipulation of biological organisms in teaching and researching as well. It is a very detailed book, that helped us to get a notion about where we are inside this big area named biosafety.
Embo Reports including Science and ethics[2] related to ethics, since we proposed a mechanism to detect biomarkers in a human serum in order to diagnose heart diseases. It is known that we cannot simply perform any sort of experiments using wild animals or even human to look for results that corroborates hypothesis of a work. But in many cases people do not know what is the correct behavior to use samples gotten from human being or from wildlife for research purposes. That is, until where we can go with research in a way that do not harm the species analysed in the experiment, mainly humans? Each country have its culture and people may have different interpretations of what actions can cause ethical problems[2]. For this reason and many others, ethics is so debated in research. There must be a common point for everyone in ethics on researching?
Crowdfunding society involvement and genetical engineering consequences. Recently, Callaway group created a plant with ability to glow in dark[3] and they were sponsored by people using a mechanism named crowdfunding. The intense interest of common people about this plant cause a general commotion in scientific mean about the consequences of spreading such modified organism unsupervised. It is not easy to predict the behaviour of such organisms into nature as well as its interactions between other organisms in environment. Since the genetical engineering grew as a big research field, many things emerged providing improvements to health (as the production of insulin), to nourishment (production of soy resistant to plagues) and energy (production of biofuels), for example, but all under restrict safety control.
During the course, we talk about such events and we comprehended biosafety not just as a list of rules that must be followed. Beyond of all restrictions applied to ensure safety, biosafety should be understood as what do you do not want to carry to your friends, relatives or all sort of people you know in order to keep them safe of any kind of risks. It is more related to avoid risks of being carried from lab to the environment than just hold them in a safe place. In the end we also concluded that we must have our critical sense always keen when dealing with science, mostly with life.
References:
- [1] Manual de biossegurança/Biosafety manual, Hirata, Mario H., Hirata, Rosário D.C., Mancini Filho, Jorge, 2012
- [2] Science and ethics, Iaccarino. M, Nature - EMBO reports vol. 14, September 2013, doi:10.1093/embo-reports/kve191
- [3] Glowing plant spark debate, Callaway, E, Nature 498, 2013 June 06, doi:10.1038/498015a
Form and Hazard
Safety
Safety forms were approved on September 24, 2013 by Evan Appleton.
Basic Safety Questions for iGEM 2013
1a. Please describe the chassis organism(s) you will be using for this project.
Species: E. coli K-12
Strain no/name: XL1-Blue
Risk Group: 1
Risk group source link: www.absa.org/riskgroups/bacteriasearch.php?genus=&species=coli
Disease risk to humans? If so, which disease? Yes. May cause irritation to skin, eyes,and respiratory tract, may affect kidneys.
2. Highest Risk Group Listed: 1
3. List and describe all new or modified coding regions you will be using in your project. (If you use parts from the 2013 iGEM Distribution without modifying them, you do not need to list those parts.) We did not use new modified coding regions, we only used new promoter regions.
4. Do the biological materials used in your lab work pose any of the following risks? Please describe.
a. Risks to the safety and health of team members or others working in the lab? Our constructs are based on E. coli and they do not offer any hazard beyond the ones intrinsic to the microorganism itself.
b. Risks to the safety and health of the general public, if released by design or by accident? The E. coli strain used in this work is not harmful to human health and has not any new genetic material that makes it harmful or gives evolutionary advantages.
c. Risks to the environment, if released by design or by accident? No new genetic material was added to the bacteria that gives it evolutionary advantages or makes it harmful if released into the environment.
d. Risks to security through malicious misuse by individuals, groups, or countries? Our engineered bacteria will be able to detect and quantify biomarkers for prognosis of cardiovascular diseases. However, several tests must be completed before we can validate the accuracy of this use. Thus, until that, our bacteria must not be used as a primary way of detecting cardiovascular disease.
5. If your project moved from a small-scale lab study to become widely used as a commercial/industrial product, what new risks might arise? (Consider the different categories of risks that are listed in parts a-d of the previous question.) Also, what risks might arise if the knowledge you generate or the methods you develop became widely available? (Note: This is meant to be a somewhat open-ended discussion question.) Since our E. coli does not carry any new coding region, even if it becomes an industrial product it will not offer any hazard beyond the ones intrinsic to the bacteria.
6. Does your project include any design features to address safety risks? (For example: kill switches, auxotrophic chassis, etc.). Note that including such features is not mandatory to participate in iGEM, but many groups choose to include them. As of now, our project does not include any design feature for safety risk, but we plan on including one in the future.
7. What safety training have you received (or plan to receive in the future)? Provide a brief description, and a link to your institution’s safety training requirements, if available. All participants of our team had to have taken a course on biosafety to work in the lab. Some had already done the course and others have done especially for this project. The course included basic rules of laboratory procedures on the containment of GMOs and the study of legislation on the subject.
8. Under what biosafety provisions will / do you work?
a. Please provide a link to your institution biosafety guidelines. http://www.icb.ufmg.br/cibio/site/wp-content/uploads/2013/03/resoluo-normativa-no-2-de-27-de-novembro-de-2006.pdf
b. Does your institution have an Institutional Biosafety Committee, or an equivalent group? If yes, have you discussed your project with them? Describe any concerns they raised with your project, and any changes you made to your project plan based on their review. Our institution does have a Biosafety Committee. We have discussed our project with the person responsible for the biosafety course in the university, Neuza Antunes. She just highlighted the importance of following the Committee’s biosafety guidelines. c. Does your country have national biosafety regulations or guidelines? If so, please provide a link to these regulations or guidelines if possible. Yes. We have an institution called CTNBio that is responsible for normalization of procedures that deals with genetically modified organisms. http://www.ctnbio.gov.br/upd_blob/0001/1620.doc.
d. According to the WHO Biosafety Manual, what is the BioSafety Level rating of your lab? (Check the summary table on page 3, and the fuller description that starts on page 9.) If your lab does not fit neatly into category 1, 2, 3, or 4, please describe its safety features. Our lab is level 1 for most of its space, and one specific room is level 2.
e. What is the Risk Group of your chassis organism(s), as you stated in question 1? If it does not match the BSL rating of your laboratory, please explain what additional safety measures you are taking. Risk Group 1. No additional safety measures were necessary in this project.
Hazard
As described in “Safety”, the risks involved with the bacteria that we used in our experiments are only the ones intrinsic to this microrganism and they are listed in the section former mentioned. To avoid these risks, we have followed biosafety guidelines. Cobalt is a toxic compound, if you are constantly exposed to large amounts of it. It can cause cardiomyopathies and nerve and thyroid problems (http://hazmap.nlm.nih.gov/hazardous-agents). As we used personal protective equipment (PPE) on its manipulation, and small quantities were used, risks involved with cobalt were understated. TMAO may cause skin, eye, and respiratory tract irritation. It is safe when used as a flavoring agent in food, but it is a strong skin and eye irritant (http://hazmap.nlm.nih.gov/hazardous-agents). As for cobalt, we used PPE and only small amounts of TMAO, what reduced its manipulation risks.
Reference:
HazMap. http://hazmap.nlm.nih.gov/hazardous-agents.
Results
- Constructs:
PSB1A3_RCNA+ YFP:

PSB1C3_TorCAD:
PSB1C3_TorCAD+RFP:
- Fluorimetric:
The results shown in here were performed as described in “Protocols”. We used Varioskan Flash Multimode Reader (Thermo Scientific™) to do the reads.
PSB1A3_RCNA+ YFP:
We have constructed the composite RCNA+YFP to detect IMA (ischemia modified albumin) in the serum of patients with cardiac risk. These patients present more IMA than normal individuals.
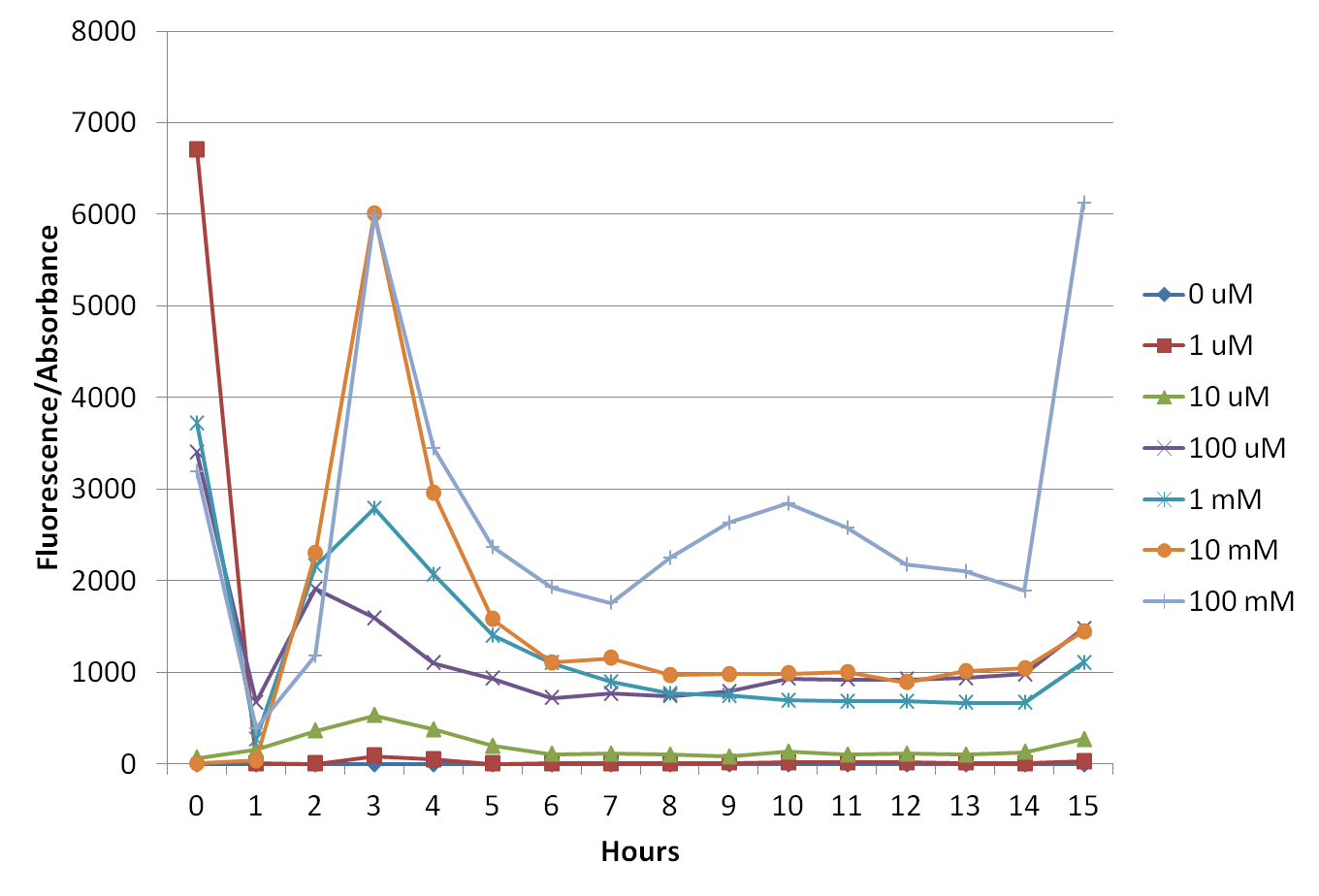
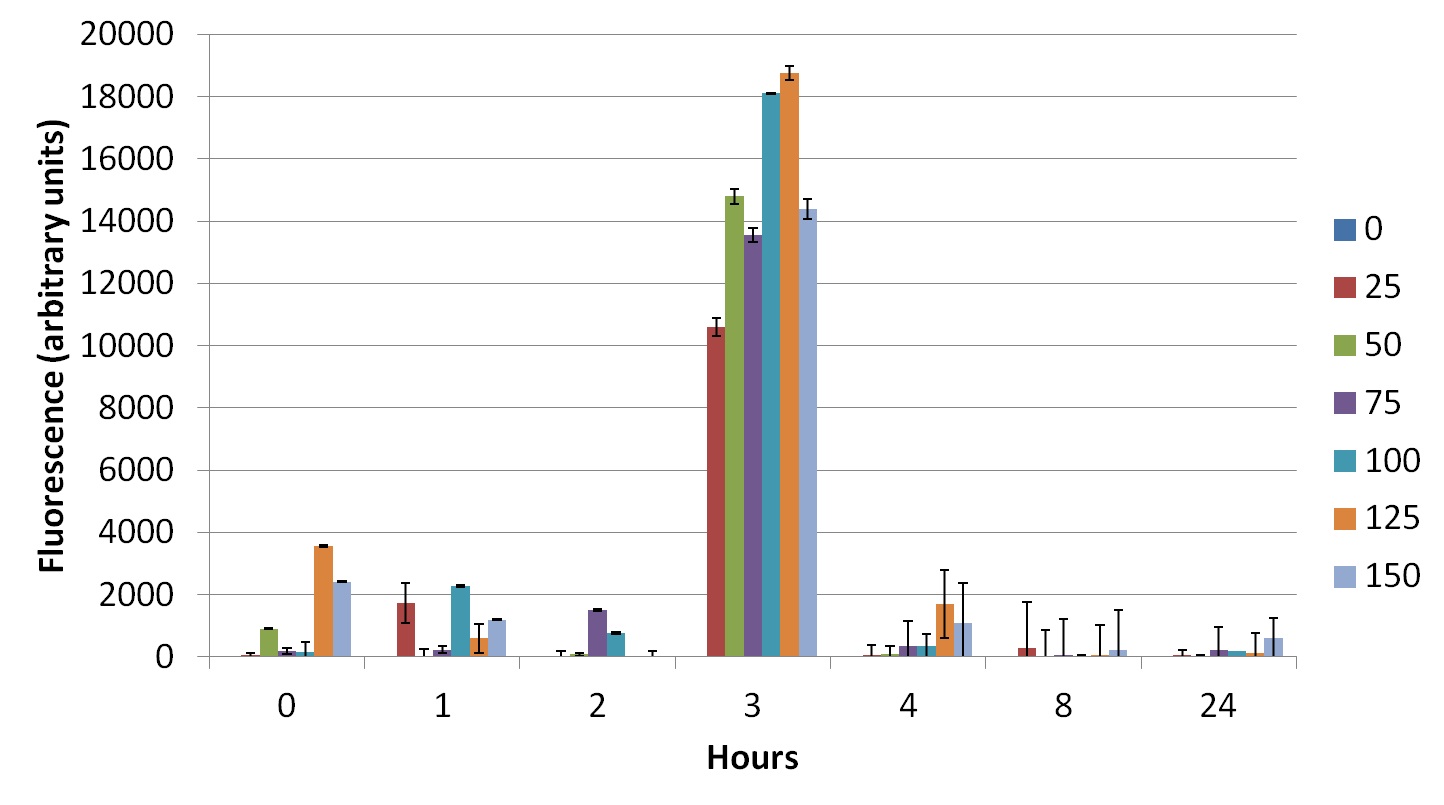
To prove that our construct works, we made some tests with transformed E. coli XL1-Blue. We added different concentrations of cobaltous chloride to bacterial cultures and measured their fluorescence (excitation: 514nm; emission: 527 nm) and absorbance (600 nm) for a certain period. The results (Figures 6 to 8) show a peak after 3 hours, during exponential phase.

As IMA binds less to cobalt than normal albumin, we expected that a serum containing more IMA would have more free cobalt than a “normal” serum. This excess of cobalt would be able to activate more RCNA promoter, which in turn would lead to expression of YFP, so we could be able to distinguish between a patient with cardiac risk from a normal patient by comparing the fluorescence generate by each serum.
First, we tested whether BSA (bovine serum albumin)would produce the expected result (more BSA, less free cobalt, less fluorescence). As can be seen on Figure 9, the result obtained meets the expected result.

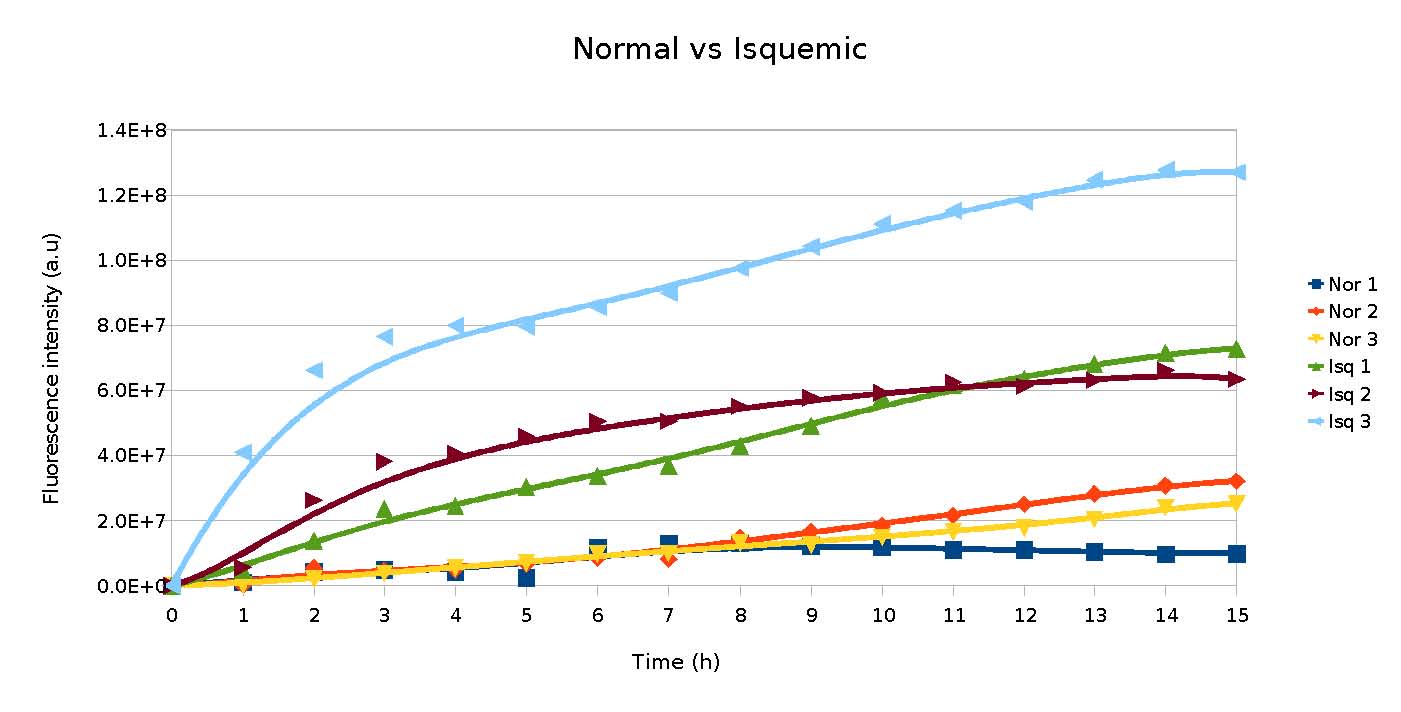
We have also added mice serum to bacteria containing the composite. These sera were from ischemic or non ischemic mice. We could see that for ischemic animals the generation of fluorescence was much higher than for non ischemic mice (Figure 10).
PSB1C3_TorCAD+RFP:
We have constructed the composite TorCAD + RFP to detect TMAO (Trimethylamine N-oxide) in the serum of patients with cardiac risk. These patients present more TMAO than normal individuals.
To prove that our construct works, we made some tests with transformed E. coli XL1-Blue. We added different concentrations of TMAO to bacterial cultures and measured their fluorescence and absorbance for a certain period. The results (Figures 11 to 12) show a peak after 3 hours, during exponential phase.

Discussion and Conclusions:
The peak of fluorescence after 3 hours (Figures 6 to 8) is probably related to the phase of growth in which bacteria are (exponential phase). At this phase, bacteria are more metabolically active, once they are dividing in a great rate, so they need to produce large amounts of proteins.
Concerning cobalt concentrations, it is likely that lower concentrations activate the promoter less than intermediate concentrations, whereas bigger concentrations might be saturating the promoter, or even causing negative feedback.
In the tests using BSA or mice sera, the results meet our model, in which more normal albumin(or BSA) leads to less free cobalt, resulting in lower fluorescence.
Our results show that the composite RCNA+YFP generates fluorescence in the presence of cobalt. Furthermore, it can be used to distinguish between ischemic and non ischemic individuals. Further characterization, including usage of samples containing human IMA (ischemia modified albumin) and normal albumin, is needed, in order to improve our composite’s documentation.
Regarding TMAO, we found a 3 hour-peak of fluorescence on the bacterial growth exponential phase once more. Higher concentration stimuli presented best results, although concentrations over 10mM seem to saturate PSB1C3_TorCAD promoter at this phase (figures 11 and 12). Further characterization using human sera is also needed for better composite’s documentation.
Our Sponsors


 "
"