Team:ATOMS-Turkiye/Lab:Protocols
From 2013.igem.org
Contents |
Protocols
Ellman’s Assay
Procedure for Quantitating Sulfhydryl Groups Using a Cysteine Standard
A. Material Preparation
• Reaction Buffer: 0.1M sodium phosphate, pH 8.0, containing 1mM EDTA • Cysteine Hydrochloride Monohydrate: M.W. = 175.6, Product No. 44889 (see Related Thermo Scientific Products) • Ellman’s Reagent Solution: Dissolve 4mg Ellman’s Reagent in 1mL of Reaction Buffer.
B. Procedure
1. Prepare a set of cysteine standards by dissolving Cysteine Hydrochloride Monohydrate at the following concentrations in Reaction Buffer:
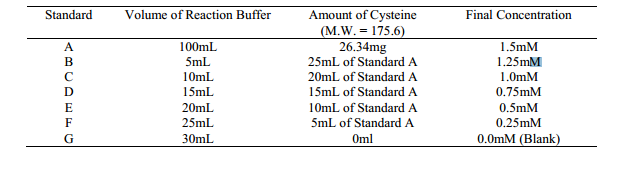
2. Prepare a set of test tubes, each containing 50µL of Ellman’s Reagent Solution and 2.5mL of Reaction Buffer. 3. Add 250µL of each standard or unknown to the separate test tubes prepared in step 2. Note: For the unknown(s), make dilutions so that the 250µL sample applied to the assay reaction has a sulfhydryl concentration in the working range of the standard curve (0.1-1.0mM is ideal). 4. Mix and incubate at room temperature for 15 minutes. 5. Measure absorbance at 412nm. 6. Plot the values obtained for the standards to generate a standard curve. Determine the experimental sample concentrations from this curve. Note: The most accurate results are obtained from the linear portion of the standard curve; i.e, the portion yielding an r2 value equal to 1.0. One or more of the high standards may exceed the linear range.
Procedure for Quantitating Sulfhydryl Groups Based on Molar Absorptivity
A. Material Preparation
• Reaction Buffer: 0.1M sodium phosphate, pH 8.0, containing 1mM EDTA • Ellman’s Reagent Solution: Dissolve 4mg Ellman’s Reagent in 1mL of Reaction Buffer.
B. Measure Absorbance
1. For each unknown sample to be tested, prepare a tube containing 50µL of Ellman’s Reagent Solution and 2.5mL of Reaction Buffer. 2. Add 250µL of each unknown to the separate test tubes prepared in step 1. As a blank, add 250µL of Reaction Buffer to a separate test tube prepared in Step 1. Note: For the unknown(s), make dilutions so that the 250µL sample applied to the assay reaction has a sulfhydryl concentration less than 1.0mM. Concentrations exceeding 1mM free sulfhydryl will result in high absorbance values and less accurate estimation of the concentration based on the extinction coefficient. 3. Mix and incubate at room temperature for 15 minutes. 4. With a spectrophotometer set to 412nm, zero the instrument on the blank and then measure absorbance of each sample. 5. Calculate the amount and concentration of sulfhydryls in the sample from the molar extinction coefficient of TNB (14,150M-1cm-1), as exemplified in Section C.
IMMUNOFLUORESCENCE
- Aspirate the medium from wells. Wash wells with PBS 1000 µl/well.
- Fix cells with prewarmed 4% Paraformaldehyde/PBS
- 500 µl /well for 15 min.at RT (add very slowly).
- Remove supernatant.
- Treat the cells for 15 min. at RT with prewarmed (37oC) TZN buffer 500 µl/well (add very slowly). Slowly mix on shaker.
- Aspirate supernatant.
- Wash wells with PBS, 750 µl/well, 5 min X 3 on shaker.
- Add Blocking Solution 500 µl/well, incubate for 1 hr at RT. Mix slowly on shaker.
Blocking Solution (f)
NGS 10%
BSA (10%) 1%
PBS-Tx 0.3%
- Aspirate off the blocking solution.
- Add 100 µl/ well 1st Ab. mixture.
- Seal the plate with parafilm, incubate at RT for 2 hr or o/n @ 4oC.
- Wash with PBS-TX 0.3%, 750 µl/well, 5 min X 1 on rocking shaker.
- Wash with PBS 750 µl/well, 5 min X 2 on rocking shaker.
- Add 100µl/ well 2nd Ab. Work in dark from this point!
- Incubate the plate at RT for 1 hr.
- Wash with PBS 1ml/ well , 5 min X 3 on rocking shaker.
- Add 300 µl/well TO-PRO-3 (1 µM) (light sensitive!)
- Incubate at RT for 15 min.
- Wash with PBS 1 ml/well, 5 min X 3 on rocking shaker.
- Add 1 drop of Prolong Gold Mounting Medium onto slides for each coverslip.
- Take out coverslip from the well. Invert and put on mounting soln. on the slide. Seal
- coverslip with nail polish.
- Let the coverslip dry. Slides can be stored at 4oC for a long time (at dark).
- Take images with confocal microscope.
Template:Team:ATOMS-Turkiye/Lab:Protocols:Western Template:Team:ATOMS-Turkiye/Lab:Protocols:PCR Template:Team:ATOMS-Turkiye/Lab:Protocols:Spin Template:Team:ATOMS-Turkiye/Lab:Protocols:Western Template:Team:ATOMS-Turkiye/Lab:Protocols:Isol Template:Team:ATOMS-Turkiye/Lab:Protocols:XCell
 "
"