Team:BIT-China/project.html
From 2013.igem.org
Overview

In modern fermentation process, cooling system plays an important role, aiming at keeping the cells in a good condition. However, that results in a great consumption of energy. According to a research carried by COFCO, the leader of bio-industry in China, once the fermentation temperature limit can be risen by 1 degree, 100 million degrees of electricity can be saved all around China. Moreover, thermal electric generation accounts for a large proportion in China. Let’s do a calculation about the effectiveness (Figure 1). The result is striking and the emission brought an enormous impact on environment such as acid rain, global warming and so on.


Inspired by this, our team managed an intelligent microbial heat regulating engine (I’MHeRE) in E.coli with the methods of synthetic biology. In this engine, two complementary systems (figure 2) were designed. The first one is the customized thermo-tolerance system used to improve E.coli’s heat-resistance and the other is the intelligent quorum regulating system used for controlling the cell density in an appropriate degree. Moreover, the engine begin to work only when it is properly to do so, ensuring the productive efficiency.
The chassis host with I’MHeRE used in the industry may make the fermentation process less depend on the cooling system and decrease the pollutant emission. On the other hand, cells can live as comfortable as in their optimum temperature, because we extend their optimum living temperature and make them live in the optimizing density. Owing to this, the high activity of the enzymes in cell could be kept in a wide range of temperature and the efficiency of microbial metabolism can be improved.
Customize thermotolerance system
Different strength promoters control different expression level of different heat shock proteins (HSPs) at different temperature to achieve the goal of Hierarchy Heat-resistant.

Figure 3: Hierarchy Heat-resistant.
 Figure 4:Over express of ASP will saddle the cell growth
Figure 4:Over express of ASP will saddle the cell growth
We found that overexpressing a protein may affect the growth of the host to a certain degree. With the help of RNA switch, they can not only be expressed in a hierarchy way, but decrease the burden of overexpressing HSPs at low temperature.


Bacteria use complex strategies to control gene expression in response to environment temperature changing. Many genes encoding heat shock proteins and virulence factors are regulated by temperature sensing RNA sequences, known as RNA thermometers (RNATs), which are located in the 5’untranslated region of the mRNA folding into secondary structure that shed RBS to influence translational efficiency in low temperature. But when temperature is increased to a certain temperature, the RNATS release RBS and the blocked genes express.
Most of gene expression responding to temperature shock measure not the temperature itself but the consequences of temperature-induced damage. Those reaction are effected by temperature indirectly. However, RNA thermometers response to temperature immediately, precisely, and controllably. So using RNA thermometers to control our heat shock proteins expression is a good choice. The RNA thermometers can be available included two types, the natural RNATS and the synthetic RNATS.
Natural RNATS
 Figure 6: ROSE RNATS
Figure 6: ROSE RNATS ROSE family
ROSE RNATS were found in numerous alphaproteobacteria and gammaproteobacteria. All known ROSE elements control the expression of small heat shock genes. ROSE-type structures are composed of two, three or four individual hairpins. The 5ʹ-most hairpins remain stable under heat shock conditions, but the 3ʹ-most hairpin that pairs with the SD sequence is only stable at low temperatures. Temperature-induced local melting in this hairpin exposes the SD sequence, facilitating ribosome binding. The SD region is occluded at 30 °C, partial melting occurs at 37 °C, whereas an increase to 42 °C facilitates the mRNA–ribosome interaction owing to full liberation of the SD and AUG start codon. This type feeds our needs. It may not initiate translation at a certain temperature completely, but the level of expression increase alone with the temperature increasing.
Four U family
 Figure 7:the structre of Four U RNATS
Figure 7:the structre of Four U RNATSAnother family of RNATs is a stretch of four uridines that pairs with AGGA in the SD sequence. FourU elements exist in the 5ʹ UTRs of several heat shock and virulence genes. It has two distinct hairpins. The first hairpin is heat stable, however the second hairpin is temperature sensitive, which hide the SD region through the fourU. The formation of the ternary translation initiation complex occurs at high (45 °C) but not at low (30 °C) temperatures.
Synthetic RNA thermometers
Obvious, RNA thermometers provide us superb genetic tools to induce or repress gene expression. However, most natural RNA thermometers are relatively large. They fold into rather complex secondary structures and have been suggested to undergo gradual conformational changes in response to changes in temperature. Therefore, we want to construct smaller and more convenient synthetic RNA thermometers to achieve the goal of hierarchy Heat-resistant regulation. Under the following principle we construct a series of synthetic RNA thermometers.
Stem stability can be influenced by (i) changes in the size and/or GC content of a perfectly matched stem and (ii) introduction of mismatches at different positions.
 Figure 8: The structures of Synthetic RNA thermometers simulated using mFold
Figure 8: The structures of Synthetic RNA thermometers simulated using mFold 
Based on literature, we know that it’s a practical and effective way to improve E.coli’s heat-resistance by importing exogenous HSPs into it. Heat shock proteins (HSPs) are a group of proteins induced by heat shock, the most prominent members of this group are a class of functionally related proteins named chaperones which are involved in the folding and unfolding of other proteins. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response heat resistance.
 Figure 9: Heat shock protein family
Figure 9: Heat shock protein family (From: http://pdslab.biochem.iisc.ernet.in/hspir/chaperone.php)
However, HSPs exist in E.coli can’t improve the heat-resistance much even when being highly expressed. As a result, we paid our attention to other hot-spring bacteria. After several strains were compared, we chose Tengcongensis MB4 which contains abundant kinds of HSPs. Here is a summary:

After further literature, we verified the function of GroEL, GroES, DnaK, DnaJ, TTE, IbpA, FliA, RpoE3 and RpoE7. Here’re the results:

Figure 10 The functional verification of HSPs
As showed above, GroEL, GroES, DnaK and FliA perform better and we chose these four to construct heat-resistance part.
Intelligent quorum regulating system

To settle the problem that when we control cell density, we use this quorum sensing device. Quorum-sensing moleculars called AHLs can cross cell membrane freely, which means that it can be used as a symbol of cell density. When the density of AHL is high enough, showing the cell density is too high, AHL will combine with a protein called LuxR and become a complex. This complex can inhibit downstream gene, TetR. The inhibition turn on the switch, and next device is activated.

Figure 11: Quorum-sensing device gene pathway

To make the apoptosis happen in a certain part of cells while the others be influenced least, we added an oscillating circuit as the moderate device. In this circuit, three substances inhibit the expression of each other, forming an oscillation as showed in figure 12.

Figure 12:Oscillator gene pathway
We can see that when the concentration of AHL reach to the threshold level, it will produce a signal which then spread to PCD device by the oscillator. The function of oscillator is to cushionthe death of cells. Meanwhile, after rounds of oscillation, the difference among cells is amplified so that a part of cells will die early and then the signal will not be produced anymore. Therefore, our destination is achieved that other cells would be alive for the cease of signal (figure 13).

Figure 13


Figure 14: MazEF system
Programmed cell death (PCD) is as an active process that results in cell suicide and is an essential mechanism in multicellular organisms. Generally, PCD is required for the elimination of superfluous or potentially harmful cells. In E.coli, there are several toxin-antitoxin systems, including mazEF, chpBIK, relBE, yefM, yoeB. The most studied among these is mazEF. MazEF system is a toxin-antitoxin system. MazF, which has RNA cleavage activity, is a kind of toxin. MazE, on the other hand, is the antidote of MazF. In nature, many bacteria will sacrifice themselves, and leave nutrition to the left ones. With this system when facing threats from dangerous environment, such as heat, lack of food and so on.
As we all know, the more E.coli the more bilogical heat would release. This is not good for cells growth as well as the productive process. So we use the PCD device to control the density.
The CI expressed in oscillation device will activate PCD device. Once CI is expressed, the expression of MazE will be inhibited. No more hexamer will be formed, and MazF will show its power. Because of mazF, the density of cell will decrease. When the density is too low that cannot activate quorum sensing system to activate osci13llation system, there will be no CI. After that, the MazE will be expressed again, which will save the cells from the hand of MazF (Figure 15).
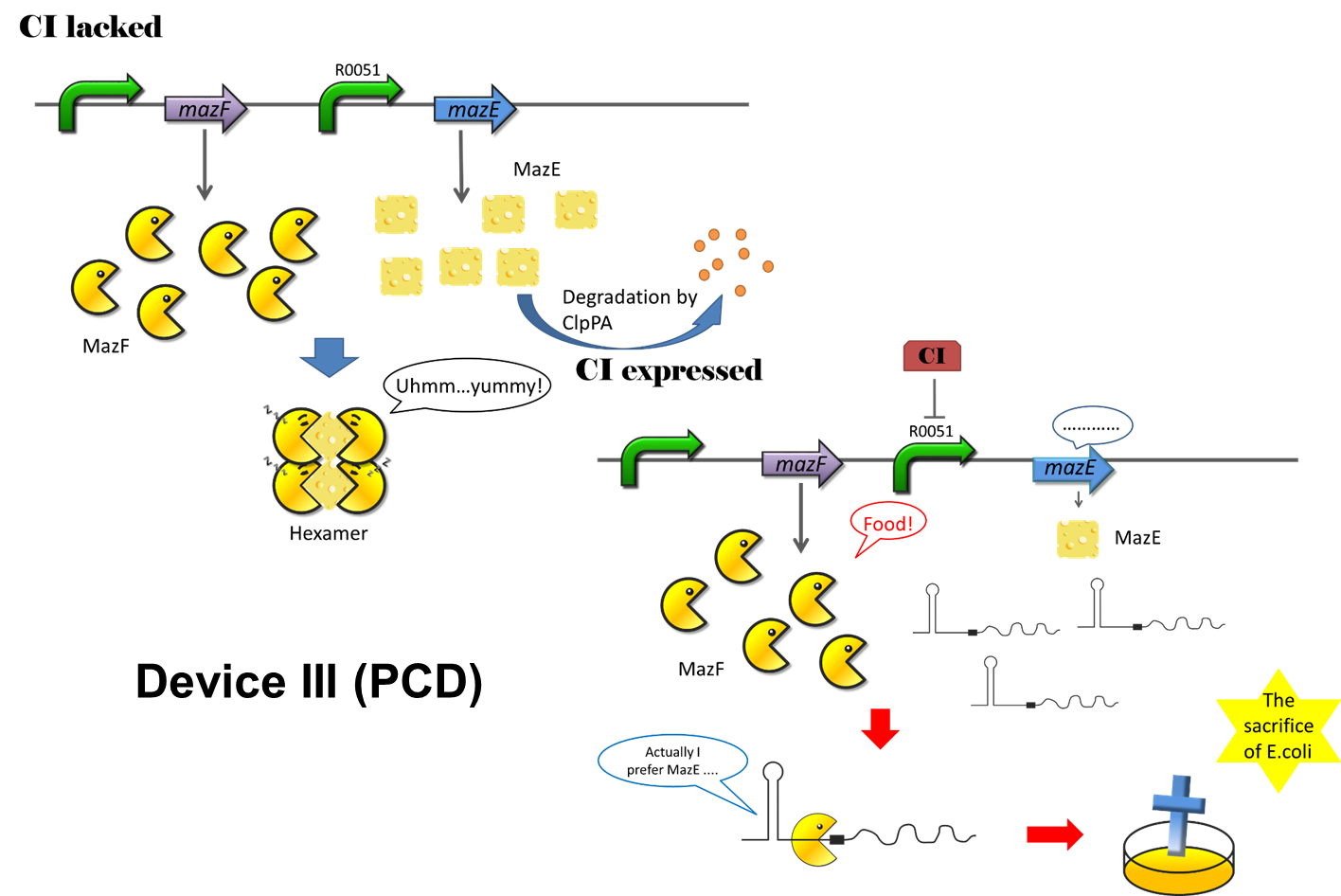
Figure 15: The program cell death system
In order to test whether this device is work, we use the T7 promoter to control the expression of CI. If the cell density decrease when CI is expressed with induction of T7 promoter, this device is successful (Figure 16).
 Figure 16: Verification of the program Cell death system
Figure 16: Verification of the program Cell death system
Parts
New Parts
BBa_K1117001
DnaK
DnaK is one of the Hsp70 proteins expressed in prokaryotes. By binding to partially peptide sequences, it can prevent proteins from aggregating and being rendered unfunctional.
http://parts.igem.org/Part:BBa_K1117001
BBa_K1117002
GroEL
GroEL(Hsp60) belongs to the chaperonin family of molecular chaperonescan. It could help proteins to fold properly with the presence of protein complex GroES(Hsp10).
http://parts.igem.org/Part:BBa_K1117002
BBa_K1117003
GroES
GroES(Hsp10) is also known as chaperonin 10 (cpn10) or early-pregnancy factor (EPF). It could help proteins to fold properly with the presence of GroEL(Hsp60).
http://parts.igem.org/Part:BBa_K1117003
BBa_K1117004
ThiF
ThiF acts like an E1 ubiquitin-activating enzyme for ThiS, which is a ubiquitin-like protein in prokaryotes. The process of ubiquitination can target denatured proteins and stimulate their degradation.
http://parts.igem.org/Part:BBa_K1117004
BBa_K1117005
J23119-K115001-DnaK
A strong constitutive promoter J23119 linked to the 42℃ RNA thermometer K115001 and heat shock protein DnaK K1117001.
http://parts.igem.org/Part:BBa_K1117005
BBa_K1117006
J23119-K115001-GroEL
A strong constitutive promoter J23119 linked to the 42℃ RNA thermometer K115001 and heat shock protein GroEL K1117002.
http://parts.igem.org/Part:BBa_K1117006
BBa_K1117007
J23119-K115001-ThiF
A strong constitutive promoter J23119 linked to the 42℃ RNA thermometer K115001 and enzyme ThiF K1117004.
http://parts.igem.org/Part:BBa_K1117007
BBa_K1117008
J23110-K115002-DnaK
A medium strength constitutive promoter J23110 linked to the 37℃ RNA thermometer K115002 and enzyme DnaK K1117001.
http://parts.igem.org/Part:BBa_K1117008
BBa_K1117009
J23119-K115001
A strong constitutive promoter J23119 linked to the 42℃ RNA thermometer K115001.
http://parts.igem.org/Part:BBa_K1117009
BBa_K1117010
J23110-K115002
A medium strength constitutive promoter J23110 combined with 37℃ RNA thermometer K115002.
http://parts.igem.org/Part:BBa_K1117010
BBa_K1117011
J23119-B0034-C0062-B0015
We change the Constitutive Promoter II of BBa_I9020 to make it more efficiency to feed our need.
http://parts.igem.org/Part:BBa_K1117011
BBa_K1117012
I719005-P0451
Inducible promoter T7 controls the expression of CI.
http://parts.igem.org/Part:BBa_K1117012
Used Parts
| NAME | DESCRIPTION |
|---|---|
| J23117 | Promoter |
| B0034 | RBS |
| C0062 | luxR repressor/activator |
| B0010 | Terminator |
| B0012 | Terminator |
| J23103 | Promoter |
| C0061 | Autoinducer synthetase for AHL |
| R0063 | Promoter |
| C0040 | tetracycline repressor from transposon Tn10 (+LVA) |
| C0012 | lacI repressor from E. coli (+LVA) |
| C0051 | cI repressor from E. coli phage lambda (+LVA) |
| R0051 | Promoter (lambda cI regulated) |
Cooperation
The initial device they designed is BBa_K1020004. During the cooperation, We noticed that the bacteria with this device had a slow growth rate. We think this is because the leakage of this promoter (J23100) was quite strong, for alkR was constitutively expressed and RFP had a high expression level even in the absence of alkanes as inducible compounds. We assumed that over-expression of alkR had a negative influence on the growth of cells. To eliminate the influence, we designed a modified device BBa_K1020005, which replaced J23100 with J23103, in order to reduce the expression level of the constitutively expressed alkR. It turned out that RFP showed almost no expression without alkanes. If we add C8 as inducible compounds, the red colour of the cells can be observed and the growth rate of cells is largely increased.

Figure 1: without alkanes as inducible compounds (BBa_K1020004& BBa_K1020005)

Figure 2: modified BBa_K1020005(with and without alkane as inducible compounds)
Safety
Organism & Experiment safety
The organisms used in our experiment conclude:
- Escherichia coli BL21(DE3)
- Escherichia coli K12
Although all of the organisms are in line with biosafety level 1 (BSL), we have treated it as a potential affection source and created following rules to minimize the danger:
- Wearing appropriate protective equipment like gloves and lab coats.
- Don’t eat any food or store it in the lab.
- Avoid wearing shorts or shoes that make your legs or toes exposed.
- Washing hands before leaving the laboratory.

Safety training
There is no Biosafety Committee at our university, only a bio-safety officer. Nevertheless, we have iGEM instructors, Dr. Chun Li and Dr. Shuyuan Guo, who trained us about the project progress regularly and is responsible for the bio-safety of our project. The general rule at our university is that every student has to take part in specific safety training before their first experiment. Further, once a year, every member of the lab is required to take a refreshment course in lab safety, bio safety, fire safety, waste management, handling gas cylinders and working with hazardous materials. All of these treatments ensure our lab safety.

Public & Environment safety
The model organisms used in our project are non-virulent strains which are commonly used in microbiology laboratories. The organism has been provided various resistance of antibiotics such as chloramphenicol, ampicillin and kanamycin. Without the circumstance which contained specific antibiotic, our organism has no advantage in the competition with the wild one. Besides, although we have improve its resistance of higher temperature(42~45℃), it can still be denatured via heat. In a word, it can work well in laboratory environment and will be eliminated through competition when exposing in the wild.

 "
"






