Team:BYU Provo/Notebook/Phage Purification/Winterexp/Period1/Exp/8.7CsClGradient
From 2013.igem.org
(Difference between revisions)
| Line 41: | Line 41: | ||
'''III) Reagants Used''' | '''III) Reagants Used''' | ||
| - | : | + | : T7 mutant phage |
: CsCl | : CsCl | ||
: dialysis tubing | : dialysis tubing | ||
| Line 65: | Line 65: | ||
: Fill the remaining space in the tube with phage suspension buffer to the top. | : Fill the remaining space in the tube with phage suspension buffer to the top. | ||
: Centrifuge at 26500 rpms (100,000 g) for 2.5 hours. | : Centrifuge at 26500 rpms (100,000 g) for 2.5 hours. | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
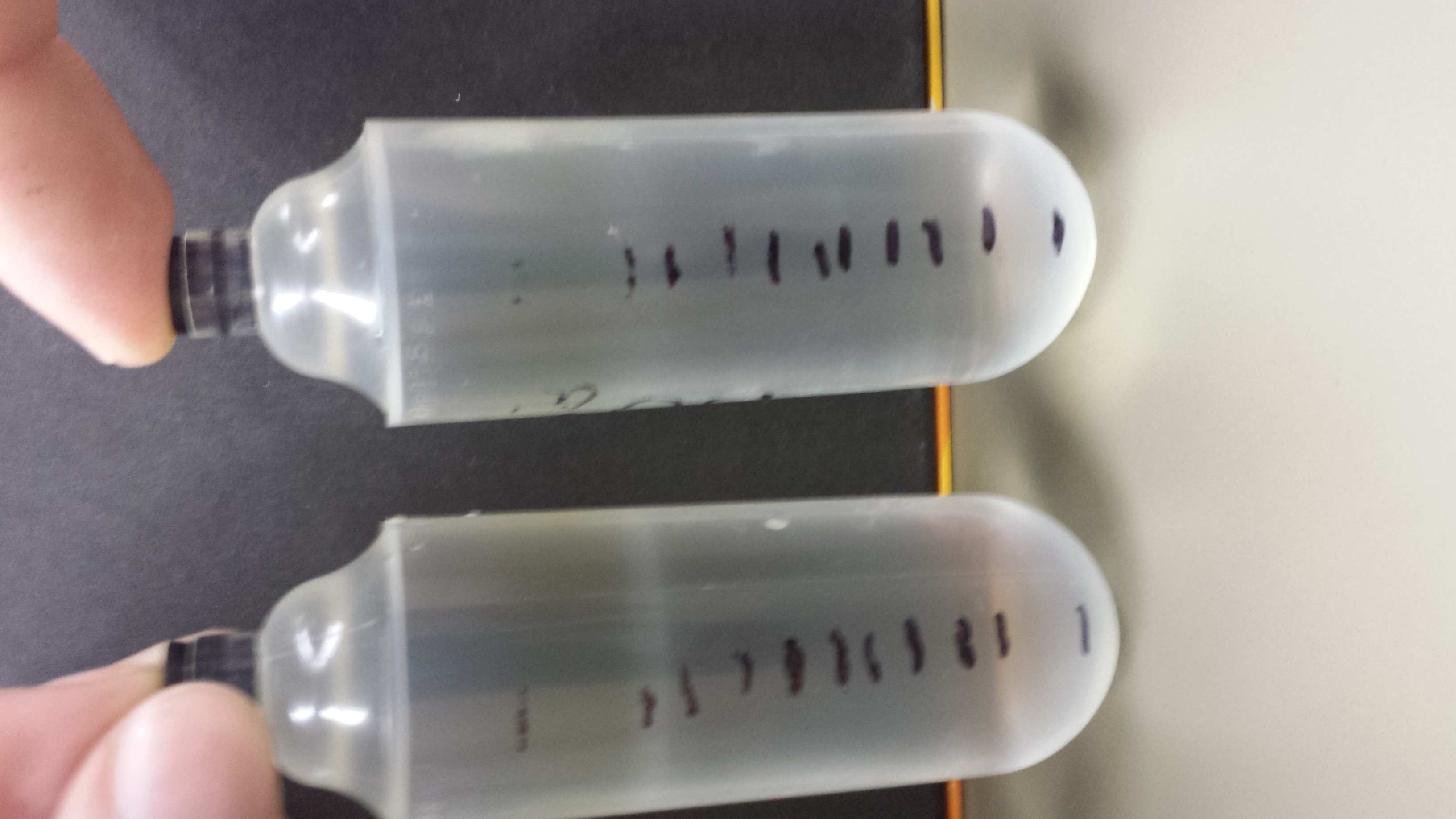
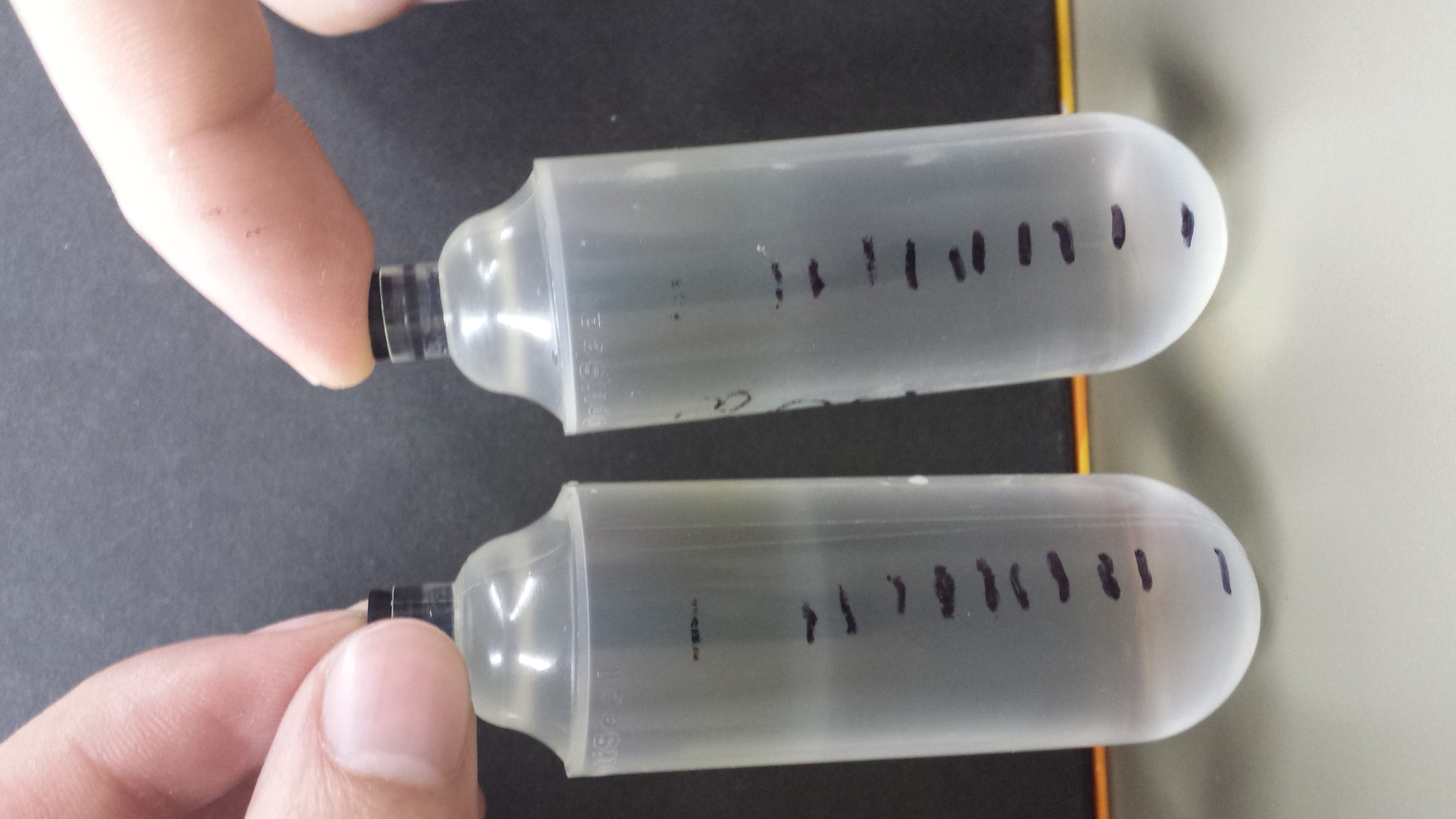
'''V) Results''' | '''V) Results''' | ||
| - | : The phage banded | + | : The phage banded ll at 1.3, allowing for no separation between wild type and mutant. The gradient will have to be run again. No phage was extracted or dialyzed. |
[[File:Phage810_1.jpg | thumb|none|alt=]] | [[File:Phage810_1.jpg | thumb|none|alt=]] | ||
[[File:Phage810_2.jpg | thumb|none|alt=]] | [[File:Phage810_2.jpg | thumb|none|alt=]] | ||
Revision as of 21:07, 23 September 2013
| Phage Purification July - August Notebook: Experiments
| ||
|
|
8.7 CsCl Gradient
I) Purpose
II) Expected Outcome
III) Reagants Used
| |
 "
"