Team:Carnegie Mellon/KillerRed
From 2013.igem.org
Enpederson (Talk | contribs) |
Enpederson (Talk | contribs) |
||
| Line 12: | Line 12: | ||
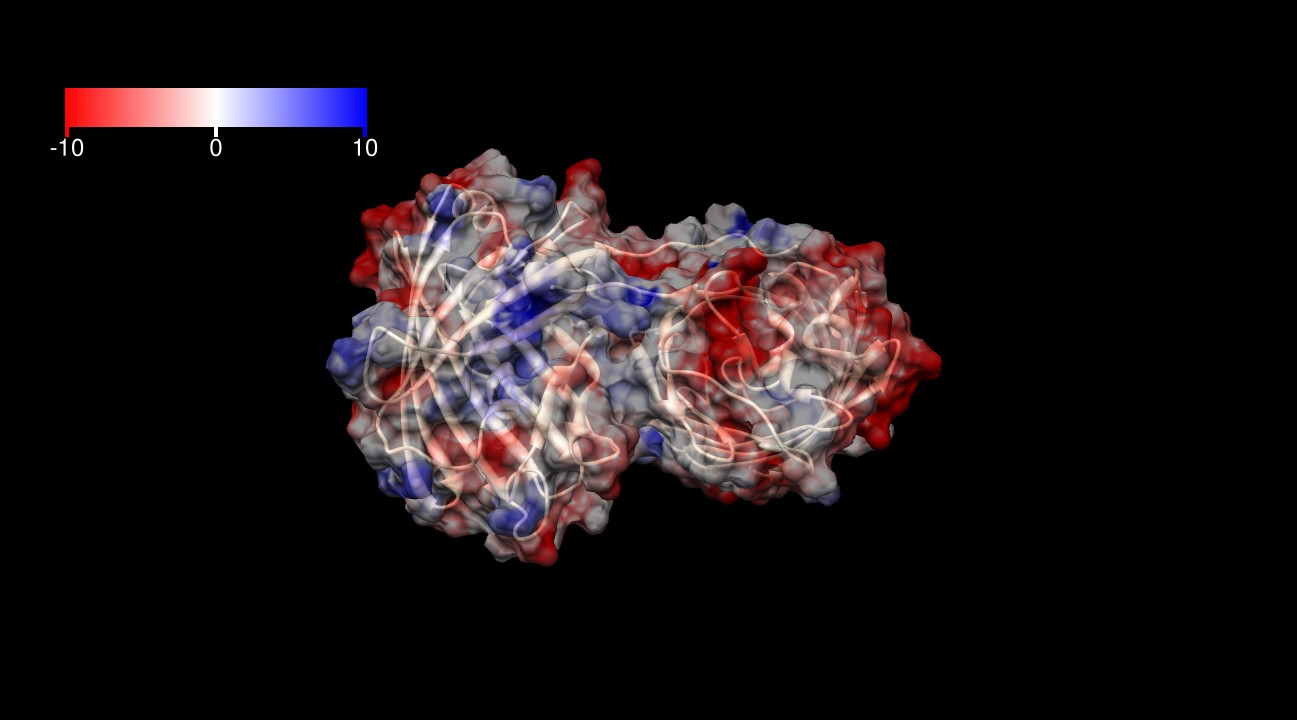
The surface of KillerRed is predominantly negatively charged. As shown in figures 3-6 | The surface of KillerRed is predominantly negatively charged. As shown in figures 3-6 | ||
</p></html> | </p></html> | ||
| - | [[Image:KillerRed ES1.jpg|thumb|600px|<b>Figure 3: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] | + | [[Image:KillerRed ES1.jpg|thumb|600px|center|<b>Figure 3: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] |
| - | [[Image:KillerRed ES2.jpg|thumb|600px|<b>Figure 4: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] | + | [[Image:KillerRed ES2.jpg|thumb|600px|center|<b>Figure 4: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] |
| - | [[Image:KillerRed ES3.jpg|thumb|600px|<b>Figure 5: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] | + | [[Image:KillerRed ES3.jpg|thumb|600px|center|<b>Figure 5: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] |
| - | [[Image:KillerRed ES4.jpg|thumb|600px|<b>Figure 6: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] | + | [[Image:KillerRed ES4.jpg|thumb|600px|center|<b>Figure 6: </b>Electrostatic surface at 1.4 Å of KillerRed dimer (from -10 kcal/(mol*e) in red to +10 kcal(mol*e) in blue). PDB ID: 2WIQ]] |
<html> | <html> | ||
</html> | </html> | ||
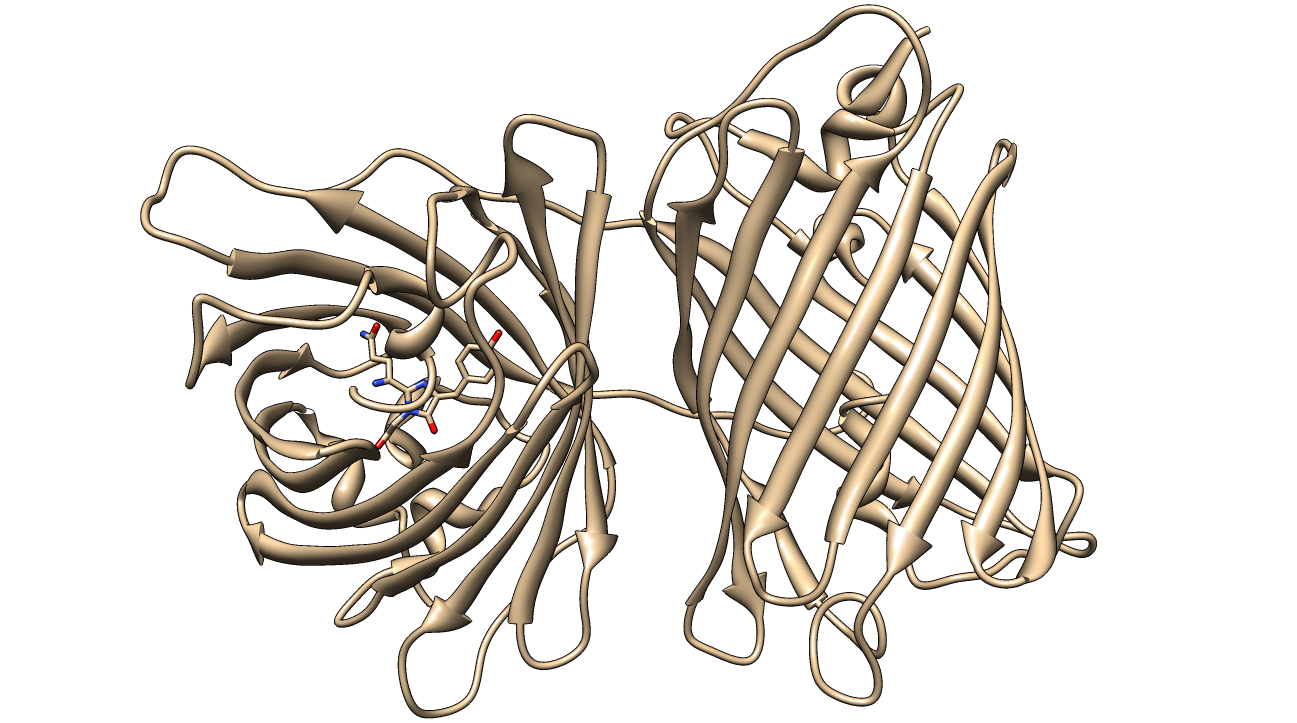
| - | [[Image:KillerRed IntFace.jpg|thumb|600px|<b>Figure 7: </b>The interface between the homodimers consist primarily of hydrophobic interactions and two hydrogen-bonding histidine residues. The histidines and their hydrogen bonds are shown. PDB ID: 2WIQ]] | + | [[Image:KillerRed IntFace.jpg|thumb|600px|center|<b>Figure 7: </b>The interface between the homodimers consist primarily of hydrophobic interactions and two hydrogen-bonding histidine residues. The histidines and their hydrogen bonds are shown. PDB ID: 2WIQ]] |
<html> | <html> | ||
</html> | </html> | ||
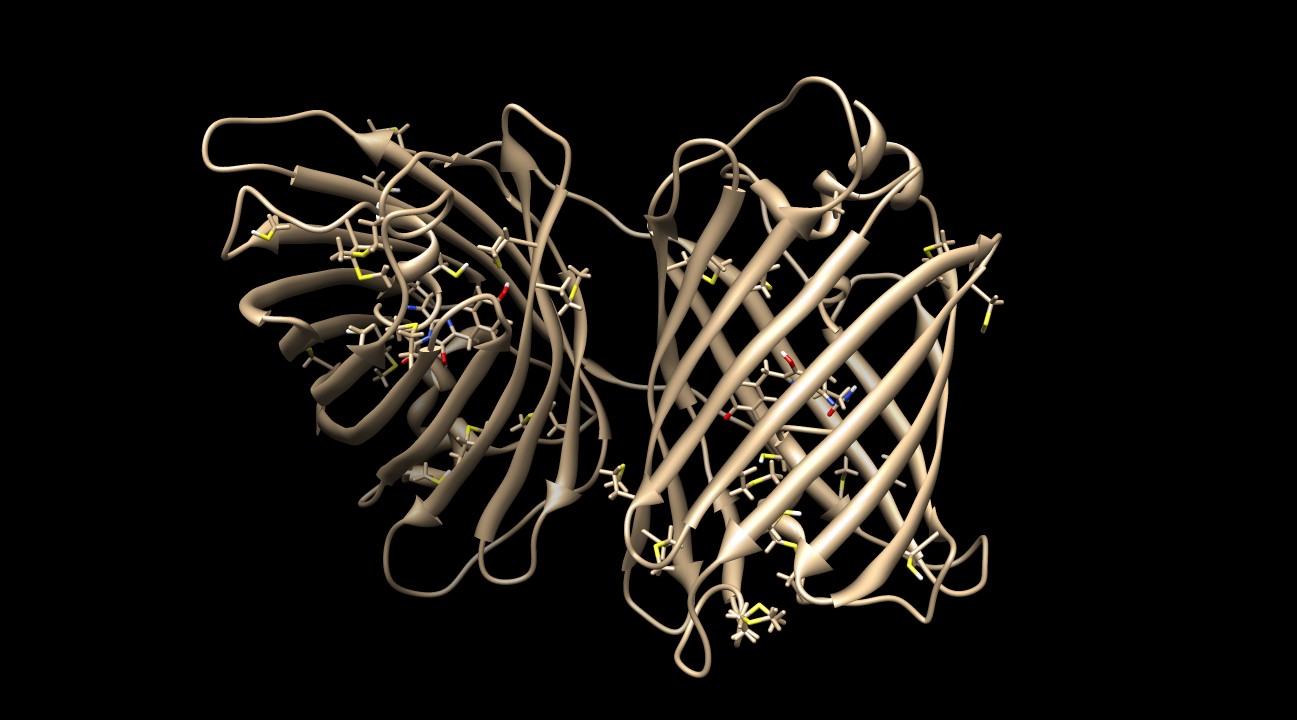
| - | [[Image:KillerRed Sulf.jpg|thumb|600px|<b>Figure 8: </b>The protein produces superoxide anion radicals, which can react with proteins and lipids to deactivate them. KillerRed contains many sulfurous residues (methionines and cysteines) that can act as antioxidants. The large volume of the sulfur atom stabilizes the radical and prevents significant damage to the protein's backbone and side chains. PDB ID: 2WIQ]] | + | [[Image:KillerRed Sulf.jpg|thumb|600px|center|<b>Figure 8: </b>The protein produces superoxide anion radicals, which can react with proteins and lipids to deactivate them. KillerRed contains many sulfurous residues (methionines and cysteines) that can act as antioxidants. The large volume of the sulfur atom stabilizes the radical and prevents significant damage to the protein's backbone and side chains. PDB ID: 2WIQ]] |
<html> | <html> | ||
</html> | </html> | ||
| - | [[Image:KillerRed wH.jpg|thumb|600px|<b>Figure 9: </b>This is the water channel that produces the superoxide anion radical when exposed to light. The ribbon structure is shown for reference. Na+ is shown in purple. PDB ID: 2WIQ]] | + | [[Image:KillerRed wH.jpg|thumb|600px|center|<b>Figure 9: </b>This is the water channel that produces the superoxide anion radical when exposed to light. The ribbon structure is shown for reference. Na+ is shown in purple. PDB ID: 2WIQ]] |
| - | [[Image:KillerRed WC.jpg|thumb|600px|<b>Figure 10: </b>This is the water channel that produces the superoxide anion radical when exposed to light. The hydrogen bonds are shown as pseudobonds and surrounding residues are shown and labeled. PDB ID: 2WIQ]] | + | [[Image:KillerRed WC.jpg|thumb|600px|center|<b>Figure 10: </b>This is the water channel that produces the superoxide anion radical when exposed to light. The hydrogen bonds are shown as pseudobonds and surrounding residues are shown and labeled. PDB ID: 2WIQ]] |
<html> | <html> | ||
<h2>References</h2> | <h2>References</h2> | ||
Revision as of 01:59, 25 September 2013
KillerRed is a red fluorescent protein that produces reactive oxygen species (ROS) in the presence of yellow-orange light (540-585 nm). KillerRed is engineered from anm2CP to be phototoxic. It has been shown that KillerRed produces superoxide radical anions by reacting with water. Superoxide reacts with the chromophore of KillerRed, causing it to become dark, which ultimately gives rise to a bleaching effect. KillerRed is spectrally similar to mRFP1 with a similar brightness. KillerRed is oligomeric and may form large aggregates in cells. Expression of KillerRed and irradiation with light may act a kill-switch for biosafety applications.
The structure of KillerRed has been analyzed and the structural basis for its toxicity has been explored. The relevant PDB IDs are 2WIQ and 2WIS for the fluorescent and dark states, respectively.
The surface of KillerRed is predominantly negatively charged. As shown in figures 3-6
References
1 Bulina, Maria E, Chudakov, Dmitriy M, Britanova, Olga V, Yanushevich, Yurii G, Staroverov, Dmitry B, Chepurnykh, Tatyana V, Merzlyak, Ekaterina M, Shkrob, Maria A, Lukyanov, Sergey, Lukyanov and Konstantin A.
A Genetically Encoded Photosensitizer
Nature Biotechnology 2006 24: 95-99. http://dx.doi.org/10.1038/nbt1175
2 Sergei Pletnev, Nadya G. Gurskaya, Nadya V. Pletneva, Konstantin A. Lukyanov, Dmitri M. Chudakov, Vladimir I. Martynov, Vladimir O. Popov, Mikhail V. Kovalchuk, Alexander Wlodawer, Zbigniew Dauter, and Vladimir Pletnev.
Structural Basis for Phototoxicity of the Genetically Encoded Photosensitizer KillerRed
Journal of Biological Chemistry 2009 284: 32028-32039. First Published on September 8, 2009, doi:10.1074/jbc.M109.054973
3Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, funded by grants from the National Institutes of Health National Center for Research Resources (2P41RR001081) and National Institute of General Medical Sciences (9P41GM103311).
4Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE.
UCSF Chimera--a visualization system for exploratory research and analysis.
Journal of Computational Chemistry. 2004 Oct;25(13):1605-12.
 "
"