Team:DTU-Denmark/Experiment2
From 2013.igem.org
Experiment 2
Contents |
Description
Measuring the production of N2O from Nitrite (NO2- ) anaerobically
We grew E. coli and P.aeroginosa anaerobically in the presence of varying concentrations of nitrite and measured the amount of nitrous oxide produced. The behavior of E. coli (untransformed cells) and P. aeruginosa is characterized while growing in the presence of NO2- in an anaerobic reaction chamber by measuring the conversion of nitrite (NO2-) to nitric oxide (NO) and nitrous oxide (N2O).
In this anaerobic experiment we add nitrite in a series of spikes to a native strain of E. coli and to a native strain of P. aeruginosa in order to test how readily nitrite is converted to nitric oxide, and subsequently to nitrous oxide. We also monitor nitrite, ammonia and nitrate concentrations before each spike. The concentrations of both gasses are measured continually, and the ammonia, nitrite and nitrate concentrations will be measured after nitrite is spiked.
Methods
The anaerobic experiment is performed in a sealed bottle, where two polarographic electrodes that will measure nitric oxide and nitrous oxide are carefully attached to the lid. The electrodes are connected to a sensor to measure the different concentrations. The bottle is placed to a magnetic stirrer on 270 rpm and 37oC.
There is one control flask, as well, containing only DM medium which will be spiked with NO2- (as will be done for the experimental flasks).
Additionally, we will measure the response of only anaerobic E. coli biomass without any NO2- added, and the same for anaerobic P. aeruginosa. This will be done in the experimental flasks prior to spiking with NO2-..
There will be 2 experimental flasks: 1. E. coli, to which spikes of NO2- will be added. 2. P. aeruginosa, to which spikes of NO2- will be added.
In addition to the continuous gas measurements, we will take samples to measure the concentration of ammonia, nitrite and nitrate at the beginning and end of each spike.
The response time of the cells to spikes of NO2- is expected to be on the order of minutes, and we will run the experiment until the solution has reached saturation with N2O.
EQUIPMENT NEEDED • 1 100 mL bottle with a lid that the electrodes can be fastened air tightly to • 1 magnetic stirrer • 1 NO probe for NO measuring • 1 N2O probe for N2O measuring • Syringes with long needles and small needles • Syringe filters with pore size 0.2μm • Stopwatch. • Modified DM Minimal Medium (Appendix 6) • E. coli overnight culture • P. aeruginosa overnight culture • Flat bottom centrifuge tubes • 2mL Eppendorf tubes • 10mL, 1mL, 200μL pipettes with tips. • MilliQ water.
All preparations with cells will be done on ice so that the cells don’t grow.
EXPERIMENTAL PROCEDURE
First, 1. Prepare DM minimal medium with ammonium chloride as a nitrogen source. Add 0.745 g NH3Cl to 1L of prepared DM medium. 2. Start overnight cultures. 3. Prepare test solutions for ammonium, nitrite and nitrate kits. 4. Label eppendorf tubes and test tubes for colorimetric samples.
Repeat the following for both E. coli and P. aeruginosa cultures: 5. Grow E. coli top10 overnight in 10mL of DM medium + NH3Cl prepared in step 1 at 37◦C. 6. Take 4mL of E. coli overnight culture and add to 400mL fresh DM medium + NH3Cl. 7. Grow the cells at 36.6◦C in 210 RPM until OD=0.35 (about 3 hours; about 5 hours for P. aeruginosa). 8. Cool down the centrifuge for 30 min at 4◦C. 9. Pellet down the 400mL culture, 3000g for 4 min at 4◦C. 10. Wash with 5 ml cold Modified DM minimal medium and centrifuge again. 11. Pour off the supernatant and resuspend the cell pellet in 100mL Modified DM Minimal medium in each centrifuge tube and pour samples together if they were made in more than one tube. 12. Measure OD of the 100 mL cell suspension and add cold Modified DM minimal medium until OD=0.3 (note the exact value). 13. Remove one aliquot of 100 mL of the OD=0.3 suspension to a bottle and keep on ice. This is the experimental flask.
For the control: 14. Remove one aliquot of 100mL of DM medium (without the added NH3Cl).
Repeat the following steps for each experiment and for the control: 15. Make the anaerobic experiment by saturating with pure N2 following the method for injecting N2 (Appendix 1), for 3 min the nitrite stock solution and 5 min the cell suspensions and controls. 16. Start the experiment by adjusting the NO and N2O electrodes in one flask at a time. 17. Put the flask on the magnetic stirrer and start with 250 rpm at 36.6◦C. 18. Remove 2 mL as the first sample t=0 colorimetric analysis. 19. Add 0.5 mL of 50mM nitrite stock solution to 100 ml of the cell suspension. Then the nitrate concentration is about 0.25mM; 3.5 mg N / L. 20. Watch the concentrations of NO and N2O, and continue when they are not changing (about 10 min). 21. Repeat steps 15-17 until the solution is exceeds the sensitivity of the sensor (1mM).
To finish gathering data: 22. Make the colorimetric measurements for nitrite, nitrate and ammonium for each of the samples collected by using the appendices 3 and 4.
Results
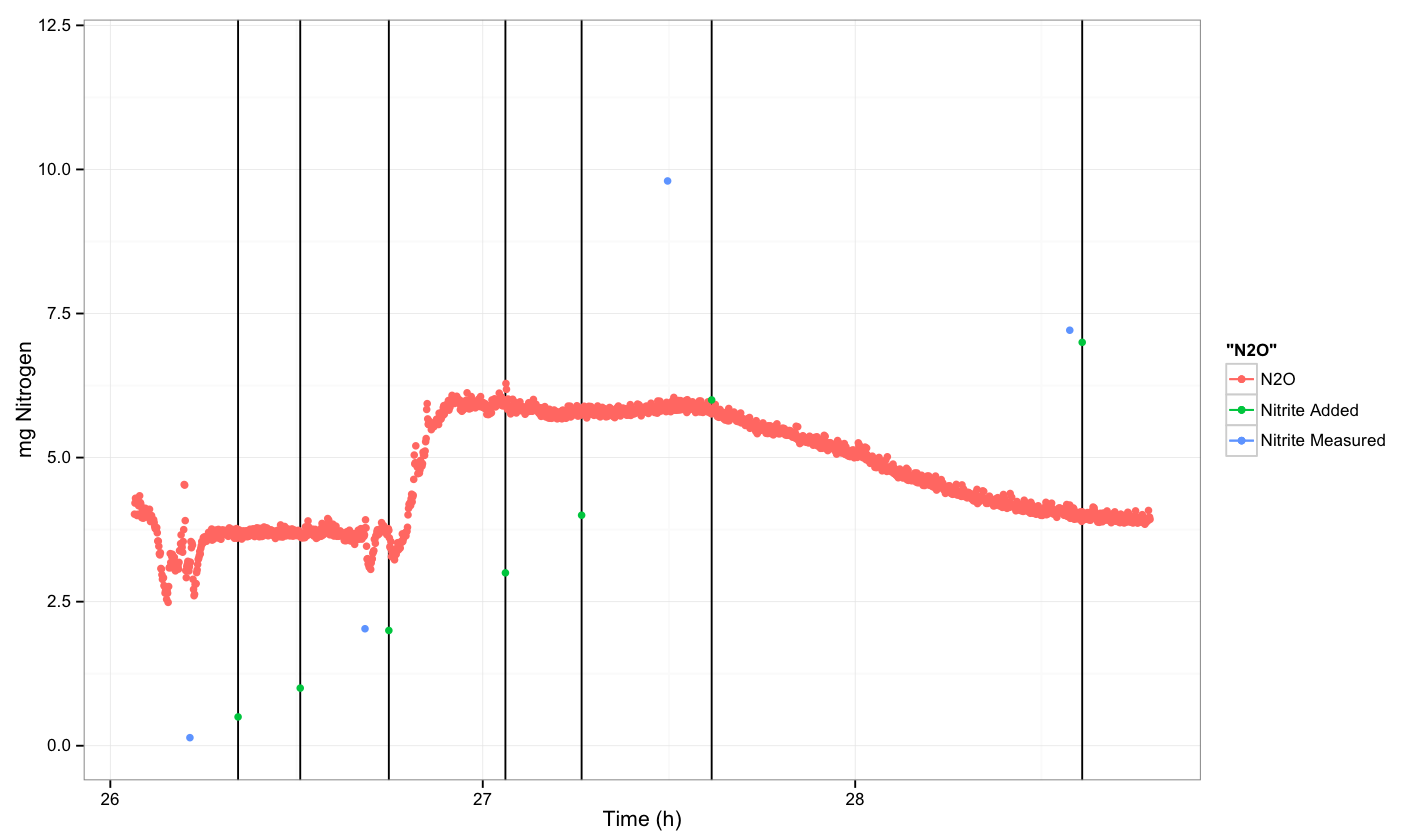
Mg of Nitrogen (as nitrite or nitrous oxide) per litre measured for varying concentrations of Nitrite added at t=0. All experiments were performed anaerobically.
 "
"